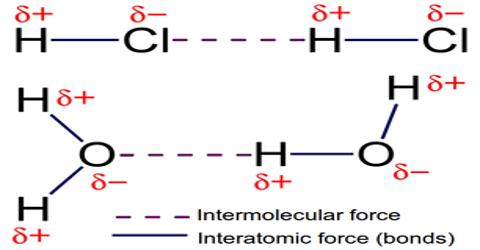
Intermolecular Forces
Intermolecular forces are defined as the set of attractive and repulsive forces that occur between the molecules as a result of the polarity of the molecules. Intermolecular forces are forces of attraction or repulsion which act between neighboring particles (atoms, molecules, or ions). They are weak compared to the intramolecular forces, the forces which keep a molecule together. We know that atoms and molecules are made up of only protons, electrons and neutrons. Of these, protons in the nucleus are positively charged, electrons are negatively charged and neutrons do not carry any charge. These are particles with very small mass. As the effect of gravitational attraction between particles of such small mass is extremely small and negligible, one can only think that the forces between particles must be electrical in nature. The melting point and the boiling point of a substance are measures of the strength of such forces. The higher the melting and boiling points stronger must be the attractive forces between particles. i.e. ions or molecules.
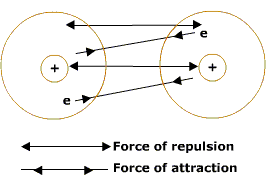
From the study of the nature of these forces it has emerged that there are different types of forces between particles. These forces are listed below:
(a) Ionic interactions
(b) van der Waals forces
- Dipole —dipole attractions
- Dipole-induced dipole interaction,
- Dispersion forces (instantaneous dipole-induced – induced dipole interaction)
- Hydrogen bonding.