J. J. Thomson, in 1898, proposed that an atom possesses a spherical shape (radius approximately 10-10 m) in which the positive charge is uniformly distributed. The electrons are embedded into it in such a manner as to give the most stable electrostatic arrangement (Figure).
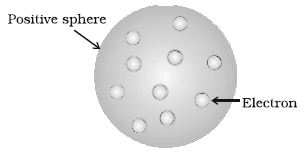
Fig: Thomson model of atom
Many different names are given to this model, for example, plum pudding, raisin pudding or watermelon. This model can be visualized as a pudding or watermelon of positive charge with plums or seeds (electrons) embedded into it. An important feature of this model is that the mass of the atom is assumed to be uniformly distributed over the atom. Although this model was able to explain the overall neutrality of the atom, but was not consistent with the results of later experiments. Thomson was awarded Nobel Prize for physics in 1906, for his theoretical and experimental investigations on the conduction of electricity by gases.