Arsenious sulphide sol (double decomposition) Preparation
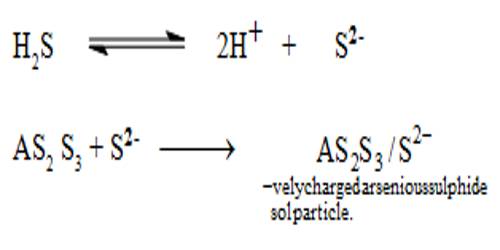
By double decomposition, a sol of arsenic sulphide is obtained by passing hydrogen sulphide through a cold solution of arsenious oxide in water. For the preparation of arsenious sulphide sole, a saturated solution of pure arsenious oxide is made in clean water by boiling in a clean flask. The solution is cooled to about 50°C and the undissolved oxide is removed by filtration and it is diluted to about three times its volume with clean water. Purified H2S is slowly passed through a clear solution. The H2S reacts to form arsenious sulphide. The solution becomes yellow and almost all the arsenious sulphide remains in the colloidal state. If any precipitate is formed it should be removed by filtration through sintered crucible or glass wool and not through filter paper. Purified H2 or CO2 or N2 is now slowly bubbled through the sol till the excess H2S is removed. A clear, quite stable sol of arsenious sulphide can thus be prepared.
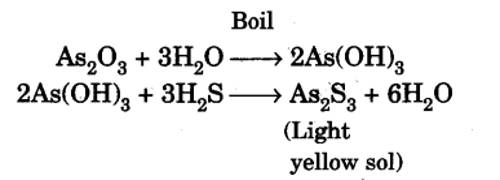
An Arsenic sulphide Sol is prepared by passing a slow stream of hydrogen sulphide gas through a cold solution of arsenious oxide. This is continued till the yellow color of the sol attains maximum intensity.
As2O3 + 3H2S→ (yellow) As2S3 + 3H2O
Excess hydrogen sulphide is removed by passing in a stream of hydrogen.
A still more convenient method is to add a small quantity of thioacetamide or thiourea to the original arsenious oxide solution in water. The thio compounds undergo slow hydrolysis forming H2S which reacts to form an arsenious sulphide. The sol thus produced contains arsenious sulphide particles even smaller than the sol prepared by the direct bubbling of H2S. While the sol made by the first process is yellow in color, the sol obtained by the action of theo compound appears yellow with a bluish tinge in transmitted light. This shows that the particles of arsenious sulphide are at least 0.01μ (10 nm).
Procedure –
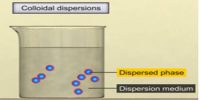
(i) The lyophilic colloids have a strong affinity between particles of the dispersed phase and the dispersion medium.
(ii) Simply mixing the dispersed phase and dispersion medium under ordinary conditions readily forms these colloidal solutions.
(iii) For example, the substance like gelatin, gum, starch, egg, albumin, etc. passes readily into the water to give a colloidal solution.
(iv) They are reversible in nature become these can be precipitated and directly converted into a colloidal state.