Types of Colloids
A colloid is one of the three primary types of mixtures, with the other two being a solution and suspension. A colloid may be defined as a substance in a peculiarly fine state of subdivision dispersed in another continuous medium giving rise to a large increase in surface area of the dispersed phase. It is a mixture that has particles ranging between 1 and 1000 nanometers in diameter, yet are still able to remain evenly distributed throughout the solution. It diffused slowly and passed through membranes very slowly and the system is not always transparent to light. Examples of this group were gum, glue, etc.
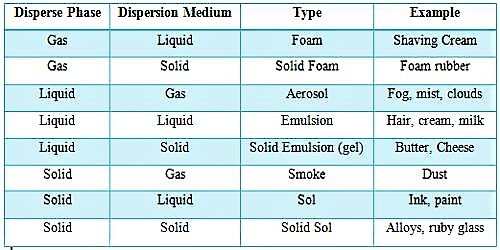
In colloids, one substance is evenly dispersed in another. Depending on the dispersion medium and disperse phase colloidal systems have been grouped into different types. The substance being dispersed is referred to as being in the dispersed phase, while the substance in which it is dispersed is in the continuous phase. The material body which is present in a state of fine discrete particles is called the disperse phase and the continuous medium in which the discrete particles are present is called the dispersion medium. It is the continuous medium, such as a gas, liquid, or solid, in which a disperse phase is distributed. The table lists some of these,
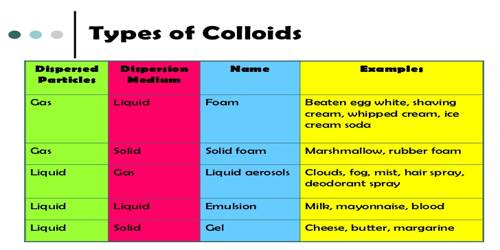
Table: Different types of Colloids
Classifying Colloids
A common method of classifying colloids is based on the phase of the dispersed substance and what phase it is dispersed in. The types of colloids include sol, emulsion, foam, and aerosol.
- Sol is a colloidal suspension with solid particles in a liquid.
- An emulsion is between two liquids.
- Foam is formed when many gas particles are trapped in a liquid or solid.
- Aerosol contains small particles of liquid or solid dispersed in a gas.
A colloid is a mixture in which one substance of microscopically dispersed insoluble particles is suspended throughout another substance. Unlike solutions, colloids do not constitute a solute dissolved in the solvent phase. Rather, the solute phase is dispersed in the solvent phase. The dispersed-phase particles have a diameter between approximately 1 and 1000 nanometers. Such particles are normally easily visible in an optical microscope, although at the smaller size range (r < 250 nm), an ultramicroscope or an electron microscope may be required. Some colloids are translucent because of the Tyndall effect, which is the scattering of light by particles in the colloid. Other colloids may be opaque or have a slight color.