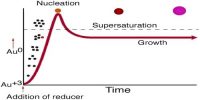
The Electrical Double Layer
The electrical properties of the colloidal state of matter and other associated phenomena may be explained in terms of the existence of an electrical double layer at the interface between two phases. The concept of the electrical double layer arises from the question of the origin of the electrical charge on colloidal particles as evidenced by the electro kinetic phenomena. The charge on the colloidal particles is supposed to be derived by adsorption of ions of electrolytes from the solution, as it is found that in the absence of traces of electrolytes most lyophobic colloids are unstable. In the case of lyophilic colloids the charge is formed by the ionization of ionizable groups present in the colloidal particle. Whether the colloidal particle will adsorb positive or negative ions will depend on the nature of surface of the particles and sometimes on the concentration of the electrolytes.
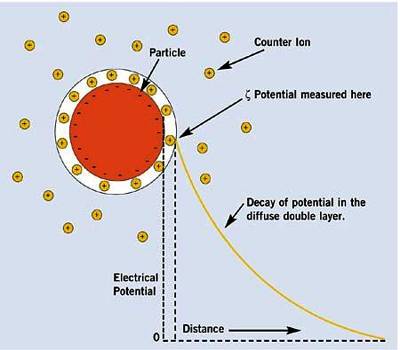
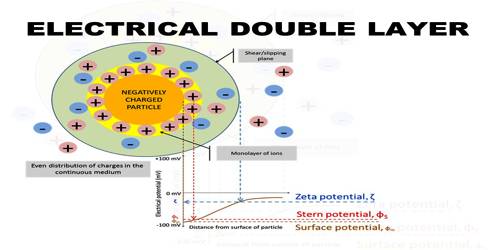
Let us consider a colloidal particle carrying a positive charge. A layer of positive ions from the electrolyte will be strongly adsorbed on the surface. As the surface as a whole is electrically neutral there will be present in the vicinity of the surface negative charges (counter ions or gegen ions) as shown in Figure. The concentration of the negative charges gradually decreases as the distance from the surface increases. The layer of positive charges on the particle and the negative charges in the suspension medium constitute what is known as an electrical double layer.
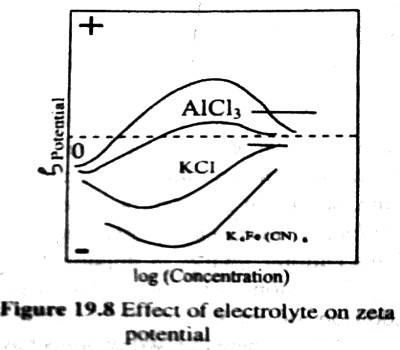
The potential difference between the surface layer and the solution is known as electrokinetic potential or zeta potential (Figure 1). The magnitude of this potential depends not only on the nature and extent of adsorption but also on the distance from the surface of the particle. The zeta potential may be modified by changing the nature and concentration of the electrolyte as shown in Figure 2. The thermodynamic potential represents the total potential of the solid phase and the liquid phase.