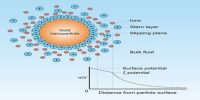
Silver Bromide Sol (double decomposition) Preparation
A very dilute solution of silver nitrate is prepared in subdued light and any suspended impurity is carefully removed by filtration. If this solution is added a very dilute clean solution of potassium bromide turbidity does not appear immediately. After a while a silver bromide sol is formed which looks bluish-white in transmitted light, indicating very small particles of silver bromide. Even without any dialysis, the sol is stable for quite some time.
Electrophoresis of silver bromide sol containing urea and guanidine were studied at pH 3-11 and pAg 3-11. The concentration of these additives was varied at the range of 0-10-1 mol/mol AgBr. No effect of these additives on the electrophoresis of silver bromide sol was observed. The conclusion was derived that these additives both does not adsorb on or react with silver bromide. It proved impossible to give the wall of the capillary a positive charge with AgNO3 solutions. Only if on the wall fresh AgBr was precipitated, he succeeded but when the precipitate was some hours old it could no longer be done.