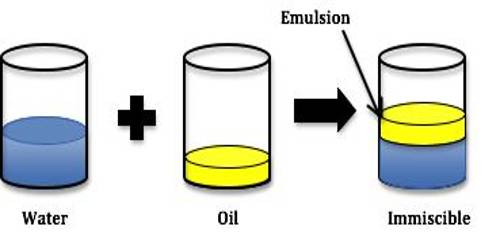
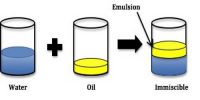
An emulsion is a dispersion of one liquid into another liquid in the form of small globules. Milk is a well-known emulsion. Many oils of animal or vegetable origin are used in medicine in the form of emulsions. Since in almost all cases water is one of the liquids and the other is an organic liquid, emulsions are classified as
(i) Oil in water (O/W), where fat globules are dispersed in water.
(ii) Water in oil (W/O) emulsions, where water globules are dispersed in oil.
Milk is a common example of the emulsion of the type O/W, where fat globules are dispersed in aqueous phase. Butter, on the other hand, is a W/O type emulsion. The term oil is an unfortunate choice but it is still being used to represent the organic phase. In general, the liquid droplets at rest or even during flow or stirred condition (except at very high field of shear) will remain spherical. Thus although emulsions are lyophobic colloids they have sonic distinctive properties.
If an emulsion is made empty by shaking two liquids like benzene and water or chloroform and water, the Emulsion on standing will quickly break and the two liquids will separate into two layers. This breaking of the emulsion is due to coalescence of the droplets. When two liquid drops touch each other, they combine to form a bigger drop, each individual droplet losing its identity completely. This is known as coalescence, to distinguish it from coagulation in which the solid colloid particles undergo aggregation. Coalescence must be prevented by some means to obtain a stable emulsion. Addition of traces of electrolytes, as in sols, cannot prevent coalescence and special reagents, called, emulsifiers, are used to give them stability.
Emulsifiers are substances which not only reduce the interfacial tension (interfacial tension may be termed as surface tension between two liquid phases) between the two liquids but form a stable coherent film around the globules and. thereby prevent coalescence (Figure). When two globules have protective films around them they cannot undergo coalescence until the film is broken.

Various types of emulsifiers are now available. They are all long-chain molecules with a hydrophilic group or lyophilic group so that one end of the molecule may be strongly adsorbed and forms a strong and coherent film of the lyophobic group around the droplets. The emulsifiers may be ionic if they are soap-like materials, or they may be non-ionic if they are esters etc. All ionic types of emulsifiers will impart an electrical charge to the emulsion.