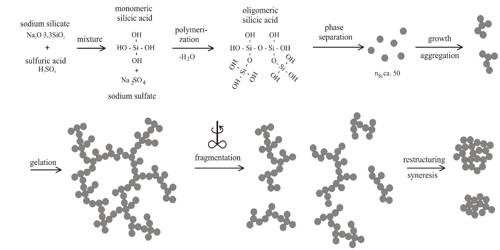
Syneresis is the contraction of a gel accompanied by the separating out of the liquid. Many gels, if allowed to stand, are found to exude some water or the dispersion medium and the gel undergoes contraction, i.e., the gel shrinks and some of the immobilized medium is given out. The phenomenon, observed by Graham himself is known as syneresis. Concentrated gels of silicic acid or dilute gels of agar-agar exhibit the phenomenon. There is no net change of volume of the gel, i.e., the volume change undergone by shrinkage is equal to the volume of the medium given out. The opposite process of syneresis is imbibition, meaning, a material that absorbs water molecules from the surrounding. Alginate is also an example of imbibition since if soaked in water, it will absorb it.
Example- Syneresis in chemistry, is the extraction or expulsion of a liquid from a gel, as when serum drains from a contracting clot of blood. Another example of syneresis is the collection of whey on the surface of yogurt. It can also be observed when the amount of diluent in a swollen polymer exceeds the solubility limit as the temperature changes.