Plastic upcycling refers to the process of transforming plastic waste into new, high-value products with a longer lifespan. This process helps to reduce the amount of plastic waste in landfills and oceans, and it also reduces the demand for new plastics, which can help to close the carbon cycle.
The carbon cycle refers to the process by which carbon moves through the environment. Carbon is released into the atmosphere through various natural and human activities, such as respiration, burning fossil fuels, and industrial processes. This carbon can then be absorbed by plants, animals, and oceans, and can be stored in soil and rock formations.
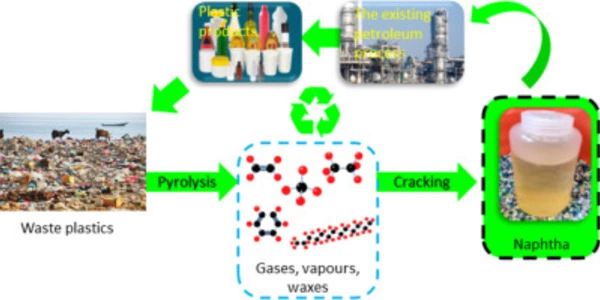
A new method for converting waste plastic into fuel and raw materials promises to close the carbon cycle at low temperatures and high yield. Used face masks, grocery bags, and food wrap contain a wealth of potentially useful raw materials. However, it has been much cheaper to continue producing these single-use plastics than to recover and recycle them.
An international research team led by the Department of Energy’s Pacific Northwest National Laboratory has now cracked the code that had thwarted previous attempts to degrade these persistent plastics. They published their findings in today’s issue of Science.
This research points to a practical new solution to closing the carbon cycle for waste plastic that is closer to implementation than many others that have been proposed.
Johannes Lercher
Low temperature and reaction control
Plastics are typically recycled by ‘cracking,’ or splitting apart the tough and stable bonds that also make them so persistent in the environment. This cracking step necessitates high temperatures, which is both costly and energy intensive.
The novel aspect of this process is the combination of the cracking step with a second reaction step that immediately completes the conversion to a liquid gasoline-like fuel with no unwanted byproducts. Alkylation catalysts are used in the second reaction step. These catalysts provide a chemical reaction that is currently used by the petroleum industry to improve gasoline octane rating.
Crucially in the current study, the alkylation reaction immediately follows the cracking step in a single reaction vessel, near room temperature (70 degrees C/158 degrees F).
“Cracking just to break the bonds results in them forming another one in an uncontrolled way, and that’s a problem in other approaches,” said Oliver Y. Gutiérrez, a study author and chemist at PNNL. “The secret formula here is that when you break a bond in our system, you immediately make another one in a targeted way that gives you the end product you want. That is also the secret that enables this conversion at low temperature.”
The research team, co-led by scientists from the Technical University of Munich in Germany, pointed to separate, recent developments by the petroleum industry to commercialize the second part of the crude oil processing process reported here.
“The fact that industry has successfully deployed these emerging alkylation catalysts demonstrates their stable, robust nature,” said Johannes Lercher, a senior author of the study and director of PNNL’s Institute for Integrated Catalysis. “This research points to a practical new solution to closing the carbon cycle for waste plastic that is closer to implementation than many others that have been proposed.”
In their study, the researchers note a limitation on their findings. The process works for low-density polyethylene products (LDPE, plastic resin code #4), such as plastic films and squeezable bottles, and polypropylene products (PP, plastic resin code #5) that are not typically collected in curb-side recycling programs in the United States. High-density polyethylene (HPDE, plastic resin code #2) would require a pretreatment to allow the catalyst access to the bonds it needs to break.
Seeing waste plastic as future fuel and new products
Petroleum-based plastic waste is an untapped resource that can be used to create useful durable materials and fuels. The plastics targeted in this study account for more than half of the 360 million tons of plastics produced globally each year. However, seeing the value in a mountain of plastic requires an innovator’s mindset, chemist’s ingenuity, and a realist’s understanding of the economics involved. These researchers are attempting to alter the dynamic by utilizing their expertise in efficiently breaking chemical bonds.
“To solve the problem of persistent waste plastic, we need to reach a tipping point where it makes more sense to collect and reuse it than to treat it as disposable,” Lercher said. “We’ve demonstrated here that we can make that conversion quickly and under mild conditions, which is one of the incentives to move forward to that tipping point.”