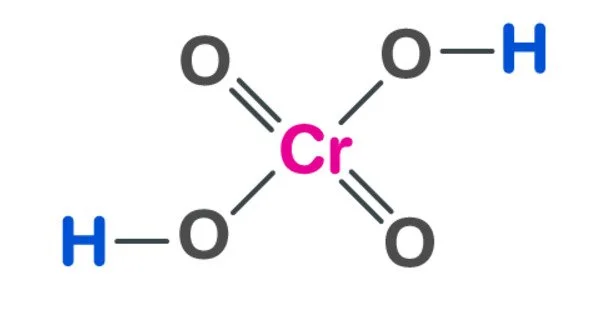
Chromic acid is an inorganic acid made up of chromium, oxygen, and hydrogen. It is a sand-like solid powder that is dark, purplish red, and odorless. It is a powerful and corrosive oxidizer. It is a strong acid when dissolved in water. Chromic acid is classified into two types: molecular chromic acid with the formula H2CrO4 and dichromic acid with the formula H2Cr2O7. Because of its hazard, it is no longer widely used and has been largely replaced by safer alternatives.
Properties
The term chromic acid refers to a mixture formed by adding concentrated sulfuric acid to a dichromate, which may contain a variety of compounds, including solid chromium trioxide. It may also refer to the molecular species H2CrO4, the trioxide being the anhydride. Chromic acid contains chromium in the +6 (or VI) oxidation state. It is a powerful and corrosive oxidizing agent as well as a moderate carcinogen.
- Appearance: It appears as dark red crystals or as an orange-red powder. It is highly soluble in water, forming a bright orange solution.
- Strong Oxidizing Agent: It is a powerful oxidizing agent, meaning it can readily accept electrons from other substances in chemical reactions.
- Acidic Nature: It is highly acidic, and its solutions are corrosive to many materials, including metals. It can react violently with reducing agents, organic materials, and flammable substances.
- Stability: It is relatively stable under normal conditions. However, it can decompose when heated or when exposed to sunlight, releasing toxic fumes of chromium trioxide.
Preparation
Chromic acid can be prepared by adding sulfuric acid (H2SO4) to a dichromate salt, typically sodium or potassium dichromate. The reaction can be represented as follows:
Na2Cr2O7 + 2H2SO4 → 2CrO3 + Na2SO4 + H2O
The resulting chromium trioxide (CrO3) is a powerful oxidizing agent and is dissolved in water to form chromic acid:
CrO3 + H2O → H2CrO4
Chromic acid is known for its ability to oxidize a wide range of organic and inorganic compounds. It has been used in various applications, including cleaning and etching metals, as a reagent in analytical chemistry, and in the production of certain dyes and pigments. However, due to its toxic nature and the availability of safer alternatives, its use has significantly declined.
Uses
Chromic acid has several industrial applications, including:
- Electroplating: It is used in the electroplating industry to create a thin layer of chromium on various metal surfaces, providing corrosion resistance and aesthetic appeal.
- Wood Finishing: It is used in wood finishing to remove old paint or varnish layers and to prepare the surface for refinishing.
- Laboratory Reagent: It is utilized as a powerful oxidizing agent in various laboratory reactions and experiments.
- Organic Synthesis: It is used for the oxidation of organic compounds in chemical synthesis, such as converting alcohols to aldehydes or ketones.
Toxicity
Chromic acid is extremely toxic and dangerous to one’s health. When it comes into contact with the skin, eyes, or mucous membranes, it can cause severe burns. Its fumes can irritate the respiratory system and cause respiratory distress if inhaled.