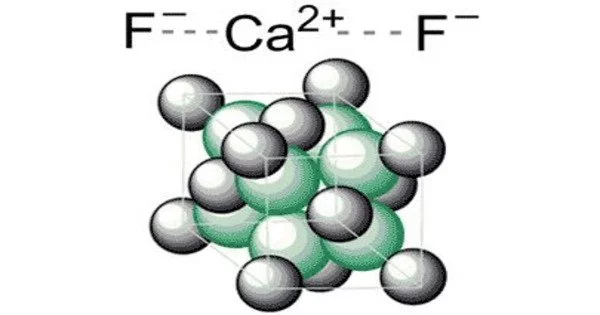
Calcium fluoride is an inorganic compound with the formula CaF2 formed by the elements calcium and fluorine. It is without a doubt one of the most well-known and valuable inorganic compounds. It is an insoluble white solid. It is found in the mineral fluorite (also known as fluorspar), which is often deeply colored due to impurities. It is used for a variety of purposes in both households and industrial plants due to its unique properties.
Calcium fluoride is widely used in industry and other applications. This chemical compound is primarily suitable for the production of hydrogen fluoride, which is used in applications such as glass etching and yeast production.
Properties
The compound has a low solubility in water. It is almost insoluble in acids and is a poor electrical conductor. The molecular weight of calcium fluoride is 78.08 g/mol and the density is 3.18 g/cm³ (20 °C). Crystals of CaF2 start to melt at the temperature of 1418 ºC and boil at 2500 ºC.
- Chemical formula: CaF2
- Molar mass: 78.075 g·mol−1
- Appearance: White crystalline solid (single crystals are transparent)
- Density: 3.18 g/cm3
- Melting point: 1,418 °C (2,584 °F; 1,691 K)
- Boiling point: 2,533 °C (4,591 °F; 2,806 K)
- Solubility in water: 0.015 g/L (18 °C); 0.016 g/L (20 °C)
- Solubility product (Ksp): 3.9 × 10−11
- Solubility: insoluble in acetone; slightly soluble in acid

Preparation
The mineral fluorite is abundant, widespread, and mainly of interest as a precursor to HF. Thus, little motivation exists for the industrial production of CaF2. High-purity CaF2 is produced by treating calcium carbonate with hydrofluoric acid:
CaCO3 + 2 HF → CaF2 + CO2 + H2O
Occurrences
It is a transparent to translucent mineral with a wide range of colors ranging from intense purple to blue green to yellow, as well as reddish oranges, pinks, whites, and browns.
CaF2 is the main source of hydrogen fluoride, a commodity chemical used to make a variety of materials. As a fluoride source, calcium fluoride in the fluorite state is very important commercially. The action of concentrated sulfuric acid on the mineral releases hydrogen fluoride:
CaF2 + H2SO4 → CaSO4(solid) + 2 HF
Applications
Calcium fluoride is used as a flux in the aluminum industry and as a source of fluorine in the production of hydrofluoric acid. In cryogenically cooled thermal imaging stages, it is also used. It can also be used as a gemstone, but its worth is low due to its lack of hardness.
Calcium fluoride is used in the production of optical components such as windows and lenses, which are used in thermal imaging systems, spectroscopy, telescopes, and excimer lasers (used for photolithography in the form of a fused lense). It is transparent to a wide range of frequencies, from ultraviolet (UV) to infrared (IR). Because of its low refractive index, it eliminates the need for anti-reflection coatings. Its insolubility in water is also advantageous. It also allows for the passage of much shorter wavelengths.
Safety
Although CaF2 is classified as “not dangerous,” reacting it with sulfuric acid produces highly toxic hydrofluoric acid. In terms of inhalation, the NIOSH-recommended concentration of fluorine-containing dusts in air is 2.5 mg/m3.