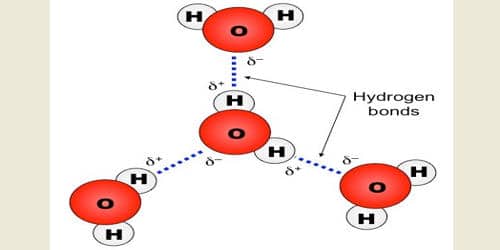
The hydrogen bond is electrostatic in nature. The strength of hydrogen bond is generally 20-40 KJ mole-1; while that of ordinary chemical bond is between 200-400 KJ mole-1. But it has important effects on the physical properties of the compounds.
For example, the compounds with hydrogen bonds have higher melting and boiling points than related compounds, but without hydrogen bonds. Although covalent compounds are generally insoluble in water, those with hydrogen bonds are soluble in water. The compounds with hydrogen bond have also higher viscosity.
A hydrogen bond is an intermolecular force (IMF) that forms a special type of dipole-dipole attraction when a hydrogen atom bonded to a strongly electronegative atom exists in the vicinity of another electronegative atom with a lone pair of electrons. Larger molecules have more space for electron distribution and thus more possibilities for an instantaneous dipole moment.
We see that H2O, HF, and NH3 each have higher boiling points than the same compound formed between hydrogen and the next element moving down its respective group, indicating that the former has greater intermolecular forces. This is because H2O, HF, and NH3 all exhibit hydrogen bonding, whereas the others do not. Furthermore, H2O has a smaller molar mass than HF, but partakes in more hydrogen bonds per molecule, so its boiling point is consequently higher.
Properties of Hydrogen Bonding –
- Solubility: Lower alcohols are soluble in water because of the hydrogen bonding which can take place between water and alcohol molecule.
- Volatility: As the compounds involving hydrogen bonding between different molecules have a higher boiling point, so they are less volatile.
- Viscosity and surface tension: The substances which contain hydrogen bonding exists as an associated molecule. So their flow becomes comparatively difficult. They have higher viscosity and high surface tension.
- The lower density of ice than water: In the case of solid ice, the hydrogen bonding gives rise to a cage-like structure of water molecules. As a matter of fact, each water molecule is linked to tetrahedral to four water molecules.
General Effects of Hydrogen Bonding –
Since intermolecular hydrogen bonds bring molecules closer together, liquids increase in density. It requires energy to disrupt these bonds, so the liquids have a higher boiling point.
Hydrogen bonds, though flexible, distort molecules into specific positions. Hydrogen bonded compounds experience greater water solubility.