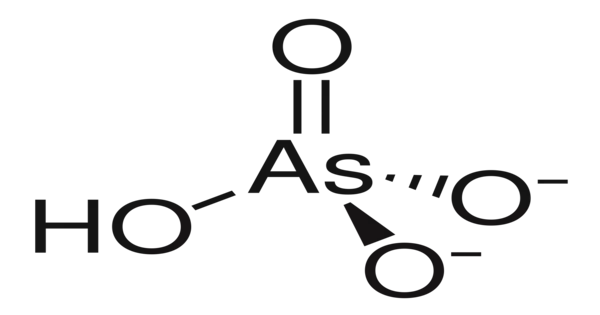
Lead hydrogen arsenate, commonly known as lead arsenate, acid lead arsenate, or LA, has the chemical formula PbHAsO4 and is largely used to control the potato beetle. It is a chemical compound containing the atoms lead, hydrogen, arsenic, and oxygen. It is a white, crystalline substance that has historically been used in agriculture as a pesticide due to its efficiency against a wide variety of insect pests.
The most common arsenical pesticide was lead arsenate. There were two main lead arsenate formulations marketed: basic lead arsenate (Pb5OH(AsO4)3, CASN: 1327-31-7) and acid lead arsenate (PbHAsO4). However, due to environmental and health issues, its use has been mostly abandoned.
Properties
- Chemical formula: PbHAsO4
- Molar mass: 347.1 g·mol−1
- Appearance: white solid
- Density: 5.943 g/cm3
- Melting point: Decomposes at 280°C
- Solubility in water: Insoluble in water; soluble in nitric acid and alkalies
Production and structure
It is usually produced using the following reaction, which leads to formation of the desired product as a solid precipitate:
Pb(NO3)2 + H3AsO4 → PbHAsO4 +2 HNO3
It has the same structure as the hydrogen phosphate PbHPO4. Like lead sulfate PbSO4, these salts are poorly soluble.
Uses
It was first employed as a pesticide against the gypsy moth in Massachusetts in 1898. It was a less soluble and less toxic alternative to the then-used Paris Green, which was about ten times more toxic. It also stuck better to the plants’ surfaces, boosting and extending its insecticidal action.
Lead arsenate was commonly employed against the codling moth and snow-white linden moth in Australia, Canada, New Zealand, the United States, England, France, North Africa, and many other places. It was mostly employed on apples, but it was also used on other fruit trees, garden crops, turfgrasses, and to repel mosquitoes. It was used as a winter treatment on lawns in southern California, together with ammonium sulfate, to destroy crabgrass seed.
Safety
Despite its pesticide efficiency, lead arsenate created serious environmental and health concerns. It resulted in the accumulation of harmful lead and arsenic residues in the soil, which could pollute crops and groundwater over time. Furthermore, exposure to lead and arsenic is linked to serious health risks.
Because of these problems, lead arsenate has been phased out and replaced by safer pesticides and pest management technologies. To safeguard both crops and the environment, modern agricultural techniques prioritize the use of ecologically friendly and less hazardous alternatives.