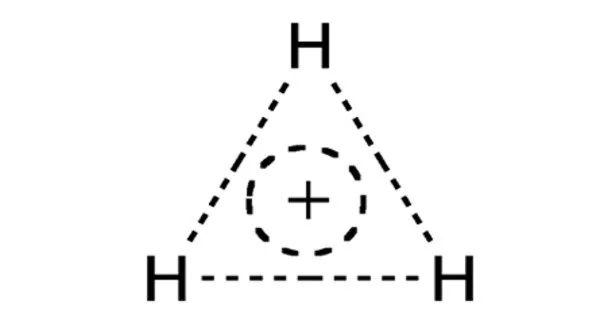
The trihydrogen cation, also known as protonated molecular hydrogen, is a positive ion (cation) composed of three hydrogen nuclei (protons) sharing two electrons. It is a molecular ion made up of three hydrogen atoms that have each lost one electron. It is the simplest molecular ion and is extremely reactive.
One of the most abundant ions in the universe is the trihydrogen cation. Because of the low temperature and density of interstellar space, it is stable in the interstellar medium (ISM). The role of H+3 in the ISM’s gas-phase chemistry is unparalleled by any other molecular ion.
The H₃⁺ ion can be formed in several ways. One common method is through the ionization of molecular hydrogen (H₂). When a high-energy electron or a proton collides with a hydrogen molecule, it can remove an electron from one of the hydrogen atoms, resulting in the formation of the trihydrogen cation:
H₂ + e⁻ → H₃⁺ + e⁻
Another way to generate H₃⁺ is through the reaction of a hydrogen atom (H) with a dihydrogen molecule (H₂):
H + H₂ → H₃⁺
Due to its positive charge and the close proximity of the three hydrogen nuclei, the trihydrogen cation is highly reactive. It readily participates in chemical reactions, especially when combined with other molecules or ions that can accept an extra proton or electron. Because of its reactivity, it plays an important role in a variety of chemical and astrophysical processes.
Because its two electrons are the only valence electrons in the system, the trihydrogen cation is the most basic triatomic molecule. It’s also the most straightforward example of a three-center two-electron bond system.
The structure and properties of H3+ have been studied using theoretical calculations and spectroscopic studies. It has a triangular geometry, with one hydrogen atom in the center and the other two forming the triangle’s ends. The positive charge is distributed across the three hydrogen nuclei.
The trihydrogen cation has been found in a variety of environments, including interstellar space, where it is involved in the chemistry of molecular clouds. It is also involved in plasma physics research and is critical in understanding chemical reactions in extreme conditions.