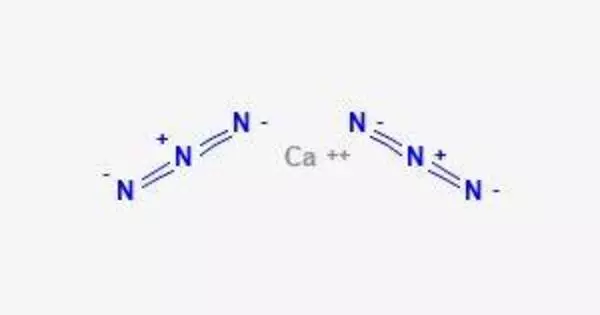
Calcium azide is a chemical compound having the formula Ca(N3)2. It is a chemical compound containing calcium and azide ions. Azide ions are made up of three nitrogen atoms covalently bound together with a negative charge, denoted by the symbol N3-.
Calcium azide is extremely sensitive to shock, heat, and abrasion. It is categorized as an explosive substance and should be handled with extreme caution. When subjected to heat or shock, calcium azide can rapidly disintegrate, releasing poisonous nitrogen dioxide (NO2) gas and perhaps causing an explosion. This is one of the reasons it is considered harmful and should be stored and handled under regulated settings.
Production
Calcium azide is a white to off-white substance that is extremely flammable and explosive. Because of its capacity to rapidly release nitrogen gas when subjected to heat or impact, it is employed in various pyrotechnic applications, such as airbag igniting systems. It can be made by distilling the reaction of hydrazoic acid and calcium hydroxide.
However, because to its extraordinary sensitivity, it is a dangerous material that should be handled with utmost caution and only by skilled individuals. The following is how calcium azide decomposes:
Ca(N3)2 → Ca + 3N2
When heated or subjected to mechanical shock, calcium azide decomposes to form calcium metal and nitrogen gas, leading to the rapid release of gas, which can be used in various applications.
Properties
- Chemical formula: Ca(N3)2
- Molar mass: 124.12 g/mol
- Appearance: colorless crystals
- Melting point: 100 °C (212 °F; 373 K) decomposes at 150 °C
- Solubility in water: 38.1 g/100 mL (0 °C)
- Solubility: slightly soluble in ethanol; insoluble in ether, acetone
Uses
Because of its explosive nature and the availability of safer alternatives, calcium azide has few practical applications. It has been utilized as a source of nitrogen gas in some airbags for quick inflation, although less dangerous chemicals are now more commonly used for this purpose.
Safety
Calcium azide is very reactive to impact and may detonate and ignite. Calcium azide is not widely available to the general population due to its extreme reactivity and explosive nature, and its usage is strictly restricted. When working with this compound, it is critical to observe safety standards and laws to avoid accidents and ensure correct handling.