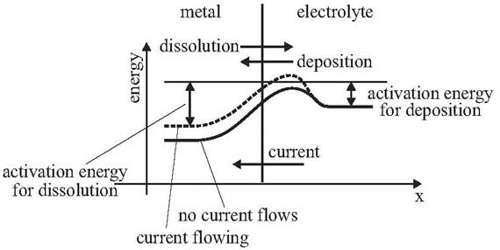
Polarization
During the electrolysis process, the product of the electrolysis on reaching the electrodes set up an emf opposite to the applies emf. The phenomenon is known as polarization.
In discussing the irreversible cell formed by dipping Zn and Cu rods in H2SO4 acid solution it was seen that when the cell is producing current H2 gas is evolved at the copper electrode. As the H2 gas bubbles accumulate on the copper electrode a virtual gas electrode which has an e.m.f. opposite to that of the Cu electrode is produced. Unless the bubbles are removed the e.m.f. of the cell will decrease as a result of the increasing opposing e.m.f. at the copper electrode. This change of e.m.f. of the cell produced by the products of electrolysis is called polarization.
For example, in the electrolysis of CuSO4solution using copper electrodes, in the beginning a very small emf is enough to start the electrolysis but soon the concentraion the concentraion of CuSO4in the neighbourhood of anode and cathode becomes different. As a result, a concentration cell is set up with emf opposite to the applied emf.
Polarization of a cell may occur,
(1) due to the change in the concentration of the ions in the neighborhood of an electrode when the cell is producing current.
(ii) when current from an outside source is passed through an electrolytic cell or,
(iii) when one or molt of the steps involved in the electrode reaction are slow.
The effect of polarization can be minimized by mechanical or chemical means.
When two clean platinum electrodes are placed in a dilute solution of hydrochloric acid and voltage is applied gradually it is found that when the voltage is low practically no current flows through the circuit, but alter a certain voltage is reached the current increased rapidly. The general behavior is shown by a curtail density (i/area) vs potential (i vs E) relationship as shown in figure.
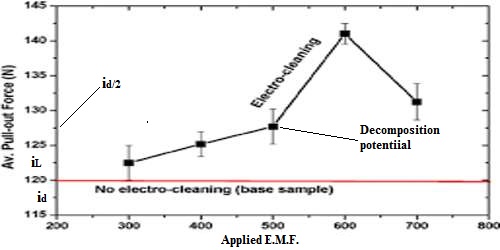
Figure: Current density plotted against applied forces.