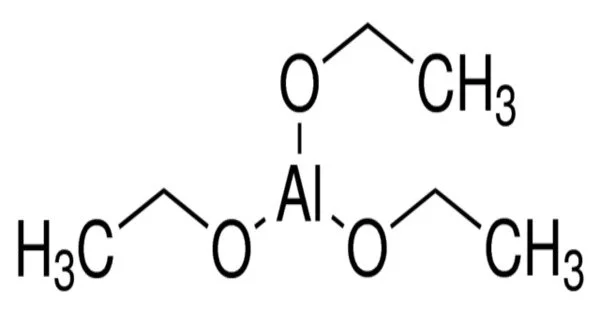
Aluminium triethoxide is a reducing agent that exists as a white powder at room temperature and standard atmospheric pressure. It is a colourless liquid that is soluble in organic solvents such as ethanol and benzene. The chemical is primarily used in industrial settings and has significantly reduced the cost of producing bimetallic aluminum catalysts.
It is used as a catalyst in organic synthesis, particularly in the preparation of esters and amides. It can also be used in the production of ceramics and as a crosslinking agent in polymer chemistry.
Properties
When aluminium triethoxide absorbs moisture from the air, it decomposes into aluminium hydroxide and ethanol. Aluminium triethoxide is slightly soluble in hot dimethyl benzene, chlorobenzene, and other high boiling point non-polar solvents.
- Chemical formula: C6H15AlO3
- Molar mass: 162.165 g·mol−1
- Appearance: White powder
- Density: 1.142 g/cm3
- Melting point: 140 °C (284 °F; 413 K)
- Boiling point: 320 °C (608 °F; 593 K)
- Solubility in water: reacts violently
- Solubility: slightly soluble in xylene, chlorobenzene
It is soluble in common organic solvents like ethanol and diethyl ether, but insoluble in water. It is sensitive to moisture and air, and it can decompose easily in the presence of water or oxygen. It is a highly reactive compound that can react with a wide range of chemicals, including water, acids, and alcohols.
Applications
Aluminium triethoxide is a polymerization catalyst as well as a reducing agent for aldehydes and ketones. Aluminium triethoxide is primarily used in the Sol-Gel Process to produce high-purity aluminium sesquioxide, a polymerization agent. At the same time, it is used as a reducing reagent, for example, to restore carbonyl compounds to alcohol.
However, it should be handled with care as it is a flammable liquid and can react violently with water, producing flammable hydrogen gas. It should be stored in a cool, dry, and well-ventilated area, away from sources of heat and ignition. Protective equipment such as gloves, goggles, and a lab coat should be worn when handling this compound.