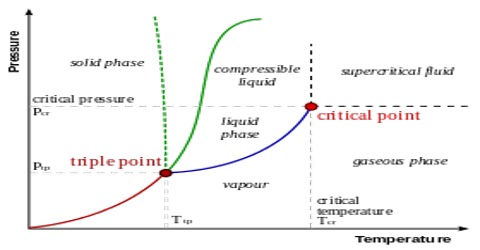
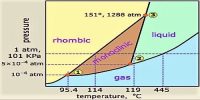
Phosphorus System
Different allotropic forms of phosphorus are well-known but for the present purpose only two forms will be considered, viz., the white and the ruby-violet. The ordinary red phosphorus is not a pure form but consists of at least four different forms of which only one is thermodynamically stable; due to the slowness of the change from one variety to the other their existence can be easily detected. Of the white and ruby-violet forms, the latter is more stable and the white can be easily transformed to the ruby-violet. Vapour pressure measurements of liquid white phosphorus and solid ruby-violet phosphorus reveal that the white form has considerably higher vapour pressure than the ruby-violet at the same temperature. The difference is so large that even at a somewhat lower temperature the liquid of the white phosphorus has a higher vapour pressure than the ruby-violet at a higher temperature. The vapour pressure of liquid white phosphorus and solid ruby-violet are shown in Table-
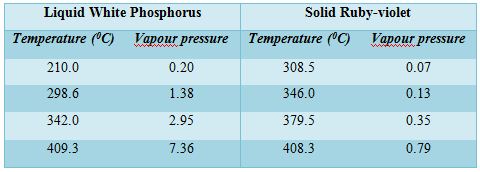
A careful study of the values of vapour pressures at different temperatures of the two forms of phosphorus reveals some interesting facts. The vapour pressure of the white form at 298.60C (liquid state) is almost double the vapour pressure of the ruby-violet at 408.3°C. Thus if white phosphorus is heated in a temperature, of say 298.60 C, it will exert a vapour pressure of 1.35 atmosphere but the vapour on condensation will give the ruby-violet form and its vapour pressure even 3460C will be only 0.13 atmosphere. The vapour of the white phosphorus at 298.60 C therefore, be heated to 346°C while the ruby-violet variety will be rapidly formed having a vapour pressure of 0.13 atmosphere only. Since the vapour pressure on the side of white form is much higher than the vapour pressure of ruby-violet form at higher temperature on the other side, a distillation is possible form a lower temperature to a higher temperature. If such a distillation is carried-out from the white form at temperatures above 260°C to the ruby-violet form at temperature between 300 – 5000C, the ruby-violet form will be in a solid state. Thus on distilling a liquid at a lower temperature to a higher temperature, a solid distillate can be obtained.