Extraction of Nickel
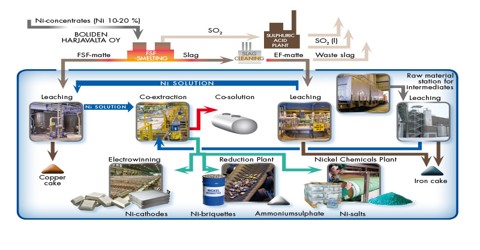
Main ore is Pentlandite; Nickel is extracted from ores through the Mond procedure, in which nickel oxides are purified throughout several steps into pure nickel metal. It is placed with hydrogen and carbon monoxide gases at 122 degrees Fahrenheit, which converts it to impure nickel. Nickel is also a magnetic metal below 653 degrees Fahrenheit. It is somewhat reactive with dilute nitric, hydrochloric and sulfuric acids. Ni is extracted from it by four processes-
(i) Condensation: by oil-froth floatation process.
(ii) Roasting
(iii) Smelting & Bessemarization: A mixture of NiS, CoS & CuS is obtained, which is called “Nickel Matt” that contains- 55% Ni. From Nickel Matt. Ni is collected by Mond or Orford’s process. It includes the following steps:
- Oxidation
- Elimination of CuO
- Reduction of NiO
- Separation of Ni
Three types of containers are used in this process:
- Reduction tower- temperature is 300°-350°C
- Sublimation tower- temperature is 500 – 800 C
- Dissociation tower- temp is 180°C
(iv) Electrolytic purification: NiSO4 is used as the electrolyte solution.
Uses of Nickel (Ni):
(i) In a finely divided form (known as raney nickel), it is used as a catalyst in the hydrogenation of oils and fats
(ii) It is used for making crucibles & laboratory apparatus
(iii) Used for electroplating
(iv) Used for making alloys: Nichrome, German silver, Monel metal etc.
Identification of Nickel (Ni): Sodium Pyroborate Decahydrate (Na2B4O7 .10H2O) is known as Borax.
Color: (Dry test) Reddish brown particulate of Nickel Sodium Orthoborate (NiNaBO3) is found.