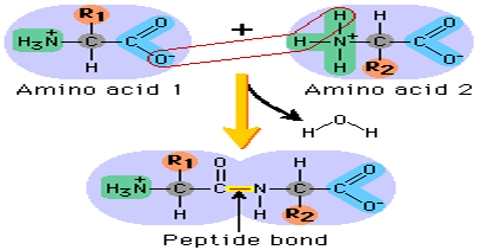
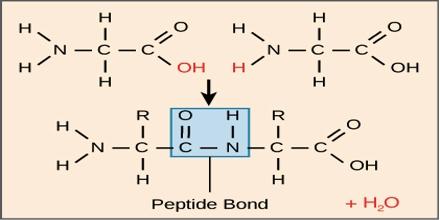
Peptide Bond: During the formation of a peptide, the amino (-NH2) group of one α – amino acid & the Carboxylic (-COOH) group of another molecule of same or different α AA get condensed with the elimination of a water molecule. This amide linkage between two molecules of AA (-CONH-) called peptide linkage or peptide bond.
It is a chemical bond formed between two molecules when the carboxyl group of one molecule reacts with the amino group of the other molecule, releasing a molecule of water (H2O). This is a dehydration synthesis reaction and frequently occurs between amino acids.
Criteria of a peptide Bond:
- It is planner
- Formation of Hydrogen bond.
In a peptide bond, four atoms- N, H, C & O and two α — carbon atoms are placed in the same plane. So it is planner. The bond between the carbonyl carbon and the nitrogen has a partial double bond character, rotation around this bond is restricted.
The bond angle of carbonyl carbon and nitrogen atom is 1200.