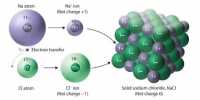
The attractive force which holds together the constituent particles (atoms, ions or molecules) in a chemical species is known as chemical bond.
Example: Two atoms of hydrogen join with each other in a chemical bond to form a molecule of hydrogen (H2).
Similarly an atom of oxygen joins with two atoms of hydrogen in a chemical bond to form a molecule of water (H2O).
There are four main types of chemical bond.
- Electrovalent or ionic bond
- Covalent bond
- Co-ordinate covalent bond
- Metallic bond
Except these bonds, there is another kind of bond which is weaker than that of real bond. These weak bonds are of three types which are-
- Vander Waal’s force,
- hydrogen bond and
- dipole-dipole attraction.