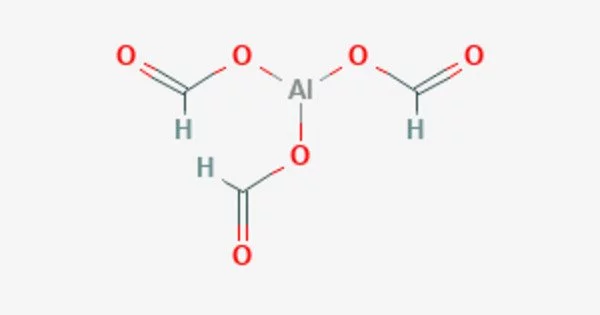
Aluminum formate [Al(HCOO)3] is a salt composed of aluminum, hydrogen, carbon, and oxygen atoms. It is the aluminium salt of formic acid. It is a white crystalline solid that is soluble in water and is commonly used as a corrosion inhibitor in industrial applications, particularly in the oil and gas industry.
It can be produced via the reaction of aluminium soaps and formic acid. It can also be used in the production of pharmaceuticals and as a reducing agent in organic synthesis. In addition, aluminum formate can serve as a source of aluminum ions in various chemical reactions.
Properties
Aluminum formate is an acidic compound and forms aqueous solutions with a pH of 3.5 to 4.5. It reacts with bases to form aluminum formate salts, such as aluminum formate hydroxide. The compound is stable under normal conditions, but it may decompose above 350°C.

- Chemical formula: C3H3AlO6
- Molar mass: 162.033 g·mol−1
- Appearance: White powder
- Solubility in water: Insoluble
- Molecular weight: 153.96 g/mol
- Density: 1.44 g/cm3 (anhydrous)
- Melting point: Decomposes above 350°C
Uses
It is a white, crystalline solid that is soluble in water and forms a basic solution. It is commonly used as a buffer in the pharmaceutical and food industries, as well as in water treatment applications. In addition, aluminum formate has been used as a precursor in the synthesis of various aluminum compounds and as a reagent in organic synthesis.
Aluminum formate is used in the manufacture of ceramic glazes, catalysts, and as a cross-linking agent for polyvinyl alcohol. It is also used in the treatment of wastewater to remove heavy metal ions and as a reagent for the determination of aluminum ions in solution.