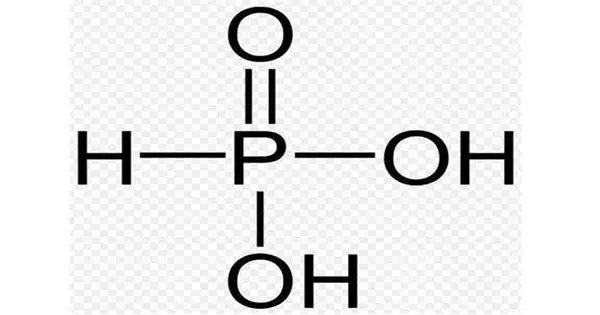
Phosphorous acid (or phosphonic acid) is the chemical compound with the formula H3PO3. This acid is diprotic (it easily ionizes two protons), not triprotic as the formula suggests. It is used as an intermediary in the synthesis of various phosphorus compounds. Phosnic acids are organic derivatives of phosphorous acid, substances with the formula RPO3H2.
The slow burning of phosphorus produces phosphorous acid in the form of a white volatile powder. Its salts are known as phosphites. It is easily produced by reacting phosphorous trichloride with water.
Properties
It is a white, crystalline solid that is soluble in water. It is a diprotic acid, meaning it can donate two protons (H+) in solution. The chemical structure of phosphorous acid consists of a central phosphorus atom bonded to three hydroxyl (OH) groups.
- Chemical formula: H3PO3
- Molar mass: 81.99 g/mol
- Appearance: white solid deliquescent
- Density: 1.651 g/cm3 (21 °C)
- Melting point: 73.6 °C (164.5 °F; 346.8 K)
- Boiling point: 200 °C (392 °F; 473 K) (decomposes)
- Solubility in water: 310 g/100 mL
- Solubility: soluble in ethanol
- Acidity (pKa): 1.1, 6.7
- Molecular shape: pseudo-tetrahedral
Phosphorous acid has strong reducing properties it tends to be converted to phosphoric acid. On being heated dry phosphorous acid disproportionates to give phosphine and phosphoric acid.
Preparation
On an industrial scale, the acid is prepared by hydrolysis of phosphorus trichloride with water or steam:
PCl3 + 3 H2O → HPO(OH)2 + 3 HCl
HPO(OH)2 could be produced by the hydrolysis of phosphorus trioxide:
P4O6 + 6 H2O → 4 HPO(OH)2
Applications
- Agriculture: It is used as a fungicide and plant nutrient. It can help control certain fungal diseases in plants and provide a source of phosphorus for their growth.
- Chemical Synthesis: It is used in various chemical reactions and synthesis processes. It can be a reducing agent and is involved in the manufacture of flame retardants, plasticizers, and other chemicals.
- Water Treatment: It can be used to reduce chlorine levels in water treatment applications.
- Electronics: It is used in the semiconductor industry for cleaning and etching silicon wafers.