Schottky defects in a Crystal
Almost all the crystals encountered in practice suffer from imperfections or defects of various kinds. An ideally perfect crystal is one which has the same unit cell and contains the same lattice points throughout the crystal.
Schottky defects defect is caused if some of the lattice points are unoccupied. The points which are unoccupied are called lattice vacancies. The number of missing positive and negative ions is the same in this case and thus, the crystal remains neutral. The existence of two vacancies, one due to a missing Na+ ion and the other due to a missing Cl- ion in a crystal of NaCl is shown in Figure.
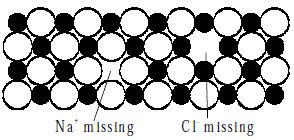
Fig: Schottky Defects in a Crystal
Schottky defects appears generally in ionic crystals in which the positive and negative ions do not differ much in size.