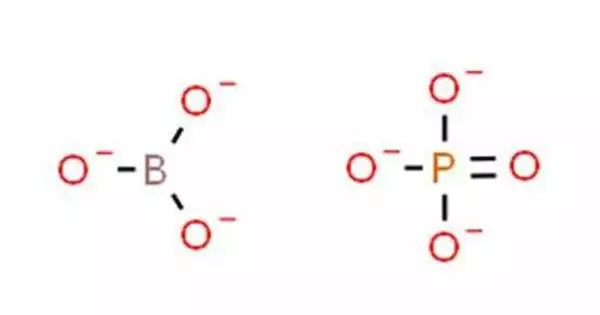
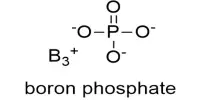
Borate phosphates are anion compounds that include both borate and phosphate anions. These are inorganic compounds with both boron and phosphorus atoms in their chemical structure. They differ from borophosphates in that the borate is linked to the phosphate via a shared oxygen atom.
These compounds frequently have unique features and are used in a variety of sectors such as chemistry, materials research, and the pharmaceutical industry. When compared to borophosphates, borate phosphates have a larger ratio of cations to borates and phosphates.
There are also organic esters of both borate and phosphate, e.g. NADH-borate.
Production
At atmospheric pressure, components are cooked together in the high temperature process. The products are anhydrous, and the synthesis of borophosphates is likely. The boron flux method includes dissolving materials such as ammonium phosphate and metal carbonate in an excess of molten boric acid.
The hue of borate phosphates can range from colorless or white to various shades of blue, green, or other colors. The presence of specific metal ions in the molecule frequently influences the hue. Some borate phosphates have luminous properties, such as fluorescence and phosphorescence, which make them valuable in optoelectronic and lighting applications.
Some borate phosphates are naturally occurring minerals, whereas others are manufactured in the laboratory. Here are a few examples:
- Wagnerite (Mg2(PO4)(BO3)): Wagnerite is a magnesium, phosphorus, and boron-containing borate phosphate mineral. It can be found in granitic rocks and metamorphic formations and is frequently utilized in the production of ceramics.
- Ludwigite (Mg2(BO2)(OH)(PO4)): Ludwigite is another mineral containing magnesium, boron, oxygen, and phosphorus. It is typically linked with other borate minerals and serves as a boron source for a variety of industrial uses.
- Synthetic Borate Phosphates: Borate phosphates, in addition to naturally occurring minerals, can also be produced in the laboratory for specific uses. These synthetic compounds can have a wide range of compositions and properties, making them useful for applications such as catalysts, ceramics, and optical materials.
The solubility of borate phosphates in water and other solvents can vary widely depending on the specific compound. Some are highly soluble, while others are relatively insoluble.
Borate phosphates are known for their diverse and often intriguing crystal structures, which can influence their physical and chemical properties. Boron and phosphorus are both elements with unique electronic configurations, and their combination in these compounds can lead to interesting chemical behavior.
Applications
Borate phosphates are used in a variety of applications, including glass manufacture, ceramics, and as catalysts in chemical reactions. Some borate phosphates are utilized in the manufacture of optical components such as lenses, prisms, and laser crystals due to their transparency and optical characteristics. Because of their biocompatibility and low toxicity, several borate phosphates have been studied for potential use in medical imaging and medication delivery systems.