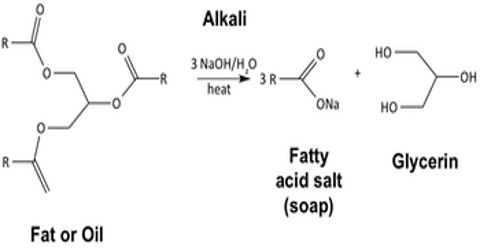
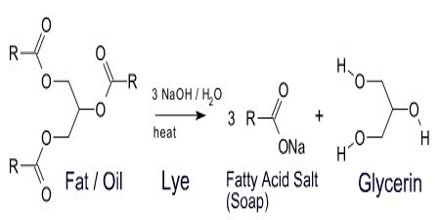
The Sodium (Na) and Potassium (K) -salts of higher fatty acids are known as “Soap”. The general formula of soap is R-COONa or R-COOK, (R = 12 – 18 C).
Production of Soap: If oils and fats are hydrolyzed by Sodium hydroxide (NaOH) or Potassium hydroxide (KOH) solution; Na-soap/K-soap, Glycerin or glycerol are produced respectively. This process is known as “Saponification.” Potassium-based soap creates a more water-soluble product than sodium-based soap, and so it is called “soft soap.” Soft soap, alone or in combination with sodium-based soap, is commonly used in shaving products.
Use:
a) Na-soap is used in washing of cloths. The carboxyllate ion of soap can transform the minute oil particles into an emulsion – thus exerting its bleaching action.
b) K-soap is highly water soluble, so it is used in production of shampoo, shaving cream etc. Toilet soap is a specialized Na-soap which contains glycerin, different colors, scents & anti-microbial agents.