The degree of ionic character in a covalent bond is determined by the electronegativity difference between the two elements bonded together. Electronegativity is the ability of an atom in a bond to attract electrons towards itself Increases left to right across a Period and it ncreases up a Group. Electronegativity difference between covalently bonded atoms produces polar bonds and molecular dipoles.
E.g. H-CI
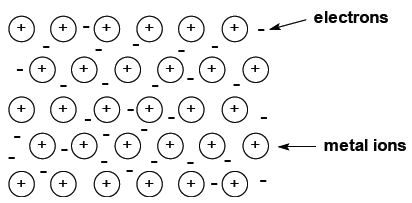
Metallic bonding The bonding in metals cannot be represented by Lewis structures. A simple picture of the structure of metals is of a regular array of positive ions with a “cloud” of delocalised valence electrons spread throughout. Metallic bonding is the strong, electrostatic attraction between each of the positive ions and the negative electron cloud.