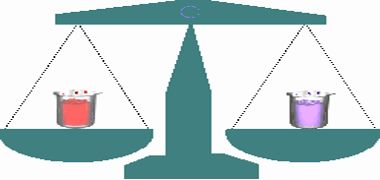
Law of Conservation of Mass states that matter can neither be created nor destroyed. The law of conservation of mass states that mass in an isolated system is neither created nor destroyed by chemical reactions or physical transformations.
This law was put forth by Antoine Lavoisier in 1789. He performed careful experimental studies for combustion reactions for reaching to the above conclusion. This law formed the basis for several later developments in chemistry. Infact, this was the result of exact measurement of masses of reactants and products, and carefully planned experiments performed by Lavoisier.