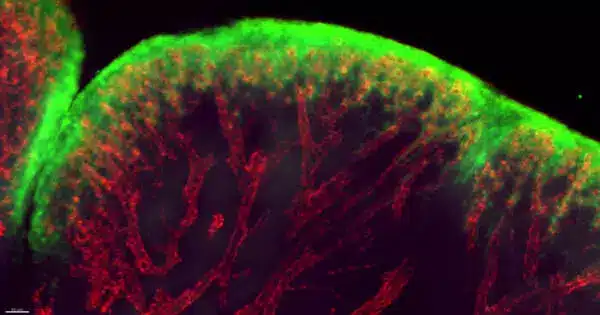
The research is the first to identify a damage response pathway that is distinct from but parallel to the traditional pathogen-induced pathway. The world is a dangerous place. Threats abound, ranging from bacteria and viruses to accidents and injuries. And nothing protects us better than our skin. The body’s largest organ is also its most seamless defense, serving as a barrier between inside and outside.
Nonetheless, the skin is not impenetrable. It is subjected to the whims of fate on a daily basis, and it tries to keep us safe by sensing and responding to these calamities. The detection of a pathogen, which activates the immune system, is a primary method. However, new research from Elaine Fuchs’ lab at Rockefeller University, published in Cell, reveals an alternative protective mechanism that responds to injury signals in wounded tissue, including low oxygen levels caused by blood vessel disruption and scab formation, and it does not require an infection to activate.
The research is the first to identify a damage response pathway that is distinct from but parallel to the traditional pathogen-induced pathway. Interleukin-24 (IL24), whose gene is induced in skin epithelial stem cells at the wound edge, is at the helm of the response. Once released, this secreted protein mobilizes a wide range of cells to begin the complex process of healing.
“IL24 is primarily produced by wound-edge epidermal stem cells, but many skin cells, including epithelial cells, fibroblasts, and endothelial cells, express the IL24 receptor and respond to the signal. IL24 becomes an orchestrator that coordinates tissue repair,” says Fuchs, who directs the Robin Chemers Neustein Laboratory of Mammalian Cell Biology and Development at the University of California, San Francisco.
Our findings provide insights into an important tissue damage sensing and repair signaling pathway that is independent of infections. We learned that the epidermal stem cells sense the hypoxic environment of the wound.
Elaine Fuchs
Hints from pathogen-induced signaling
Scientists have long understood how host responses protect our bodies from pathogen-induced threats: somatic cells recognize invading bacteria or viruses as foreign entities and initiate a number of defense mechanisms with the help of signaling proteins such as type 1 interferons. But how does the body react to an injury that may or may not involve a foreign invader? If we cut a finger while slicing a cucumber, for example, we know right away because there is blood and pain. On a molecular level, however, it is unclear how the detection of injury leads to healing.
While type 1 interferons rely on the signaling factors STAT1 and STAT2 to regulate the defense against pathogens, previous research by the Fuchs lab had shown that a similar transcription factor known as STAT3 makes its appearance during wound repair. Siqi Liu, co-first author in both studies, wanted to trace STAT3’s pathway back to its origin.
IL24 stood out as a major upstream cytokine that induces STAT3 activation in the wounds.
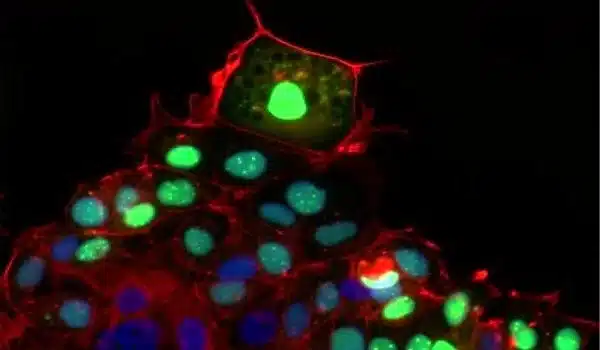
Microbe-independent action
In collaboration with Daniel Mucida’s lab at Rockefeller, the researchers worked with mice under germ-free conditions and found that the wound-induced IL24 signaling cascade is independent of germs. But what injury signals induced the cascade? Wounds often extend into the skin dermis, where capillaries and blood vessels are located.
“We learned that the epidermal stem cells sense the hypoxic environment of the wound,” says Yun Ha Hur, a research fellow in the lab and a co-first author on the paper.
When blood vessels are severed and a scab forms, epidermal stem cells at the wound’s edge are depleted of oxygen. This level of hypoxia is a red flag for cell health, and it triggered a positive feedback loop involving transcription factors HIF1a and STAT3 to boost IL24 production at the wound edge. As a result, a variety of cell types expressing the IL24 receptor worked together to repair the wound by replacing damaged epithelial cells, healing broken capillaries, and generating fibroblasts for new skin cells.
The researchers demonstrated that by changing oxygen levels, they could regulate Il24 gene expression in collaboration with Craig Thompson’s group at Memorial Sloan Kettering Cancer Center.
After determining the origin of the tissue-repair pathway in epidermal stem cells, the researchers investigated wound healing in mice genetically modified to lack IL24 functionality. The healing process was sluggish and delayed in the absence of this key protein, taking days longer than in normal mice to completely restore the skin.
They hypothesize that IL24 is involved in the injury response in other body organs with epithelial layers that serve as a protective sheath. Recent research has found elevated IL24 activity in epithelial lung tissue from patients with severe COVID-19 and colonic tissue from patients with ulcerative colitis, a chronic inflammatory bowel disease.
“IL24 could be working as a cue to signal the need for injury repair in many organs,” Hur says.
Linked by function and evolution
“Our findings provide insights into an important tissue damage sensing and repair signaling pathway that is independent of infections,” Fuchs explains.
An investigation at UT Southwestern Medical Center with evolutionary biologist Qian Cong revealed that IL24 and its receptors have close sequence and structural homology with the interferon family. Though they may not always work in tandem, IL24 and interferons are evolutionarily related and bind to receptors on the surface of cells that are close to each other. The researchers believe that these signaling molecules are descended from a common molecular pathway that dates back thousands of years.