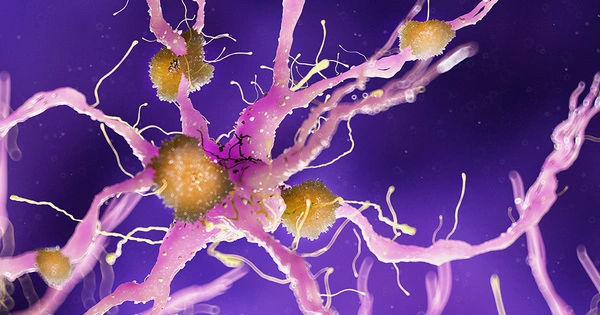
Tens of billions of neurons—specialized cells that process and transmit information via electrical and chemical signals—are found in a healthy human brain. They communicate between different parts of the brain as well as between the brain and the muscles and organs of the body. Alzheimer’s disease disrupts this communication between neurons, resulting in functional loss and cell death. Scientists developed a model to investigate the role of RNA splicing defects in Alzheimer’s disease, revealing neuron hyperexcitability-induced degeneration and toxicity.
For decades, researchers have puzzled over Alzheimer’s disease, a neurodegenerative disorder, but treatments to stop or reverse the disease’s effects on the brain have remained elusive. St. Jude Children’s Research Hospital researchers recently completed an important piece of the puzzle by developing a mouse model that more closely resembles the disease in humans than previous models. The findings were published today in the journal Nature Aging.
The researchers used their new model to investigate how RNA splicing defects contribute to neurodegeneration in Alzheimer’s disease. RNA splicing is a process that involves the removal of non-coding genetic sequences and the joining of protein-coding sequences.
“RNA splicing is an essential step between transcription and translation,” said corresponding author Junmin Peng, Ph.D., St. Jude Departments of Structural Biology and Developmental Neurobiology and the Center for Proteomics and Metabolomics, who led the research. “It is particularly important in the brain because we know the brain has more cellular diversity than any other organs in the body and splicing is believed to be an important process for generating protein diversity.”
We’ve characterized the potential contribution of RNA splicing machinery to neuron excitatory toxicity in Alzheimer’s disease from the initial behavior to cell biology and now to the molecular mechanism.
Junmin Peng
Previous research by Peng and colleagues revealed that a specific component of the RNA splicing machinery known as the U1 small nuclear ribonucleoprotein (snRNP) forms aggregates in the brains of Alzheimer’s patients. The U1 snRNP complex is required for RNA splicing.
Peng and his colleagues have now demonstrated that U1 snRNP dysfunction contributes to neurodegeneration, opening up new avenues of research for Alzheimer’s treatment. The study discovered that RNA splicing dysfunction caused by U1 snRNP pathology contributes to neurodegeneration.
“Our previous work demonstrated that the U1 snRNP is a type of aggregate in the brain that forms tangle-like structures – but that is only descriptive; we didn’t understand the mechanisms that link this pathology to the disease phenotype until now,” Peng explained.
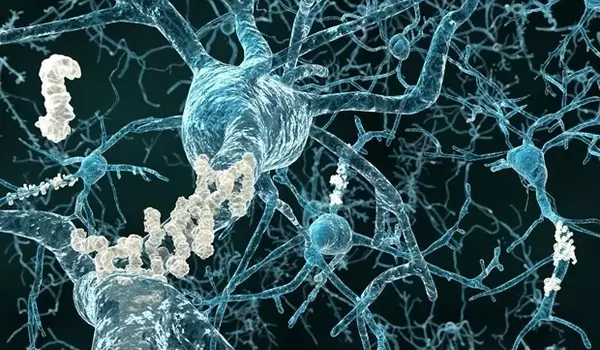
Unique model links RNA splicing defects with neuronal hyper-excitability
The researchers created a novel mouse model of RNA splicing defects called N40K-Tg. The scientists observed basic neurodegeneration when they deregulated the splicing machinery, but they wanted to understand why that was the case.
“Splicing machinery is so essential, and creating a model to study it in the lab was a real challenge,” Peng said. “We were able to create a model of splicing dysfunction that occurred only in neurons. This model demonstrates splicing dysfunction that causes neuronal toxicity as well as cognitive impairment.”
Inhibitory neuron activity keeps the brain from overheating. When inhibitory neuron activity is suppressed, neurons become more active, but this can be toxic. The new mouse model had a significant impact on synaptic proteins, particularly those involved in inhibitory neuron activity, according to the researchers.
“Excitatory toxicity is critical because it is already known in the field of Alzheimer’s disease,” Peng said. “People recognized that neurons become super excited even 20-30 years ago, and now we find that the splicing machinery may be contributing to the excitatory toxicity observed in Alzheimer’s patients.”
RNA splicing defects and β-amyloid aggregation combined
One hallmark of Alzheimer’s disease is the presence of aggregates of β-amyloid and tau in the brain. Peng’s previous work revealed that U1 snRNP forms aggregates in the brain as well, but scientists were unable to study the role of the U1 snRNP function in disease until they developed a model that perturbed U1 snRNP function causing RNA splicing defects.
The researchers crossed their mouse model with one for -amyloid to better understand how the RNA splicing defects behave in the context of -amyloid aggregation. The two types of toxic insults work together to remodel the brain’s transcriptome and proteome, deregulate synaptic proteins, and hasten cognitive decline.
“We’ve characterized the potential contribution of RNA splicing machinery to neuron excitatory toxicity in Alzheimer’s disease from the initial behavior to cell biology and now to the molecular mechanism,” Peng said. This crossed mouse model more closely resembles Alzheimer’s disease in humans than previous models and may be useful for future Alzheimer’s research.