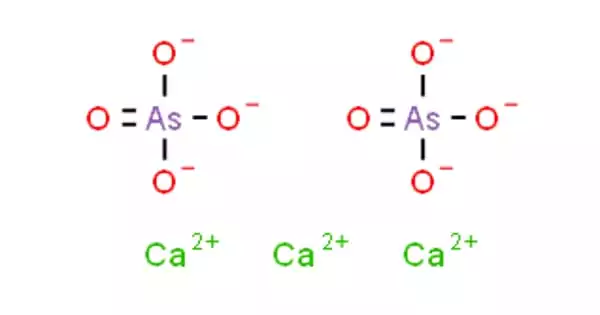
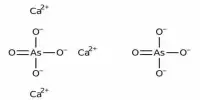
Calcium Arsenate is an inorganic chemical with the formula Ca3(AsO4)2. It comes in the form of a white powder. It is a colorless salt that was once employed as a pesticide and germicide. It is only marginally soluble in water. It is more hazardous than lead arsenate because it is more soluble in water.
Calcium arsenate hydrates include rauenthalite Ca3(AsO4)2•10H2O and phaunouxite Ca3(AsO4)2•11H2O. It is hazardous when inhaled or ingested. It’s an insecticide and a germicide.
Properties
Calcium arsenate is a foliar-based insecticide that is now banned in most countries. It is moderately soluble in water and is environmentally persistent. It is highly toxic to mammals if ingested and is a neurotoxin. It is also a probable carcinogen.
- Molar mass: 398.072 g/mol
- Appearance: white powder
- Odor: odorless
- Density: 3.62 g/cm3, solid
- Melting point 1,455 °C (2,651 °F; 1,728 K) (decomposes)
- Solubility in water: 0.013 g/100 mL (25 °C)
- Solubility in Organic solvents: insoluble
Preparation
Calcium arsenate is commonly prepared from disodium hydrogen arsenate and calcium chloride:
2 Na2H[AsO4] + 3 CaCl2 → 4 NaCl + Ca3[AsO4]2 + 2 HCl
It was created in big vats in the 1920s by combining calcium oxide and arsenic oxide. 1360 metric tons were produced in the United States in 1919, 4540 in 1920, and 7270 in 1922. The composition of commercially available calcium arsenate differs depending on the producer. A common composition is 80-85 percent Ca3(AsO4)2, a basic arsenate with a 4CaO component. As2O5 in the presence of calcium hydroxide and calcium carbonate.
Use as a herbicide
It was originally widely used as a herbicide and pesticide. In 1942 alone, 38,000,000 kilos were reported to have been produced, primarily for cotton crop protection. DDT was developed as a result of its high toxicity.
Regulation
Calcium arsenate use is now prohibited in the United Kingdom, and it is carefully regulated in the United States. It is now the active ingredient in Mallinckrodt’s TURF-Cal, and it is one of the few herbicides – used mostly to reduce Poa annua and crabgrass – that inhibits earthworm activity. Its label claims that it will “reduce and hinder earthworm activity and survival,” however it is only advised for major earthworm infestations in places like golf course greens.
Toxicity and regulation
Calcium arsenate is extremely hazardous, causing both carcinogenesis and systemic health problems. The Occupational Safety and Health Administration has established a tolerable exposure limit of 0.01 mg/m3 for an eight-hour time-weighted average, whereas the National Institute for Occupational Safety and Health suggests a limit five times lower (0.002 mg/m3).