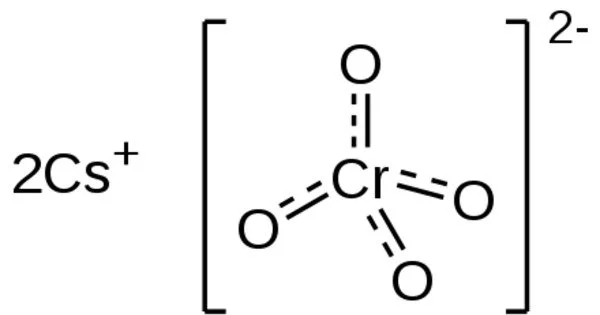
Cesium chromate is a caesium and hexavalent chromium chemical compound. It has the formula Cs2CrO4 and is an inorganic compound. It is a yellow crystalline solid that crystallizes in the orthorhombic system and is the caesium salt of chromic acid. It is used to make vacuum tubes.
Its primary application in the past was the generation of caesium vapour during the fabrication of vacuum tubes. It is currently only used as a precursor to other compounds of academic interest. It is oxidized, soluble in water, but insoluble in alcohol. It is used in the reaction with silicon, boron, or titanium to produce caesium vapour.
Properties
- Chemical formula: Cs2CrO4
- Appearance: Yellow crystalline solid
- Density: 4.237 g/cm3
- Melting point: 954 to 961 °C (1,749 to 1,762 °F; 1,227 to 1,234 K)
- Solubility in water: 45.50 g/100 g (25 °C)
- Crystal structure: orthorhombic

Preparation
Caesium chromate is mainly obtained from the reaction of chromium(VI) oxide with caesium carbonate, wherein carbon dioxide gas is evolved:
CrO3(aq) + Cs2CO3(aq) → Cs2CrO4(aq) + CO2(g)
Alternatively, salt metathesis between potassium chromate and caesium chloride can be performed:
K2CrO4(aq) + 2 CsCl(aq) → Cs2CrO4(aq) + 2 KCl(aq)
Finally, caesium dichromate (itself derived via salt metathesis from ammonium dichromate) yields the chromate following alkalinisation with caesium hydroxide:
Cs2Cr2O7(aq) + 2 CsOH(aq) → 2 Cs2CrO4(aq) + H2O(ℓ)
It is oxidizing, sealed and preserved, soluble in water, insoluble in alcohol. Heating and reacting potassium chromate and cesium chloride solution, evaporating and concentrating, filtering, cooling and crystallizing, centrifuging and drying are all used to make it.
Applications
Cesium chromate is used to create cesium vapor through a reaction with silicon, boron, or titanium, which is then used in the final stages of vacuum tube manufacturing. It is used in the manufacture of phototubes.
Caesium chromate was previously used in the final stages of vacuum tube manufacturing. Caesium vapour was produced in this study by reacting caesium chromate with silicon, boron, or titanium as reducing agents. After that, the vapour was added to the tube to react with and remove any remaining gases, such as nitrogen and oxygen.