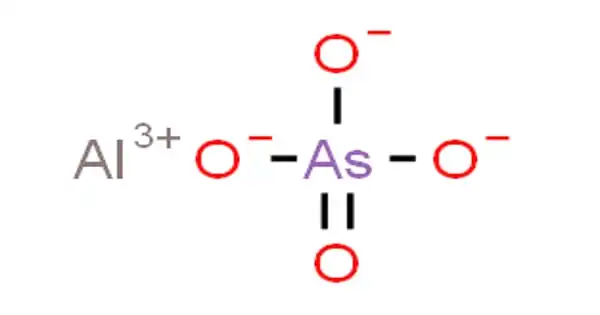
Aluminium arsenate, often known as AlAsO4, is an inorganic chemical having the formula AlAsO4. It is most usually found in the form of an octahydrate. It is a colorless solid formed by the interaction of sodium arsenate with a soluble aluminum salt. It is most usually found in the form of an octahydrate.
Mansfieldite, a mineral containing aluminium arsenate, occurs naturally. Alarsite, in its anhydrous form, is a fumarolic mineral that is extremely rare. The hydrothermal process is used to create a synthetic hydrate of aluminium arsenate. Al2O3.3As2O5.10H2O is the formula.
Properties
It is a colorless solid formed by the interaction of sodium arsenate with a soluble aluminum salt. Mansfieldite, a mineral containing aluminium arsenate, occurs naturally. Under oxidizing soil conditions, arsenate is the primary inorganic species of arsenic and is retained in soils via adsorption processes.
- Chemical formula: AlAsO4
- Molar mass: 165.901 g/mol
- Appearance: white crystals
- Density: 3.25 g/cm3
- Melting point: 1,000 °C (1,830 °F; 1,270 K)
- Solubility in water: insoluble
- Crystal structure: hexagonal
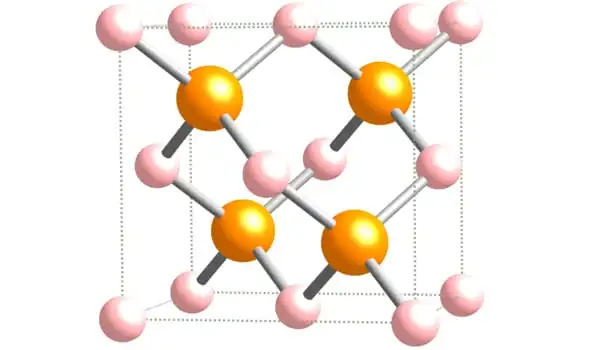
Crystal System
Heating different samples at different temperatures was used to modify aluminium orthoarsenate. It was possible to obtain both amorphous and crystalline forms. The solubility product for aluminium arsenate with the formula AlAsO4.3.5H2O was determined to be 10-18. It has the same -quartz structure as gallium arsenate and boron arsenate. The high-pressure version comprises a rutile-type structure with six-coordinated aluminium and arsenic.
Toxicity
Arsenic is a chemical element with the atomic number 33 and the symbol [As]. It is a deadly metalloid with numerous allotropic forms, including yellow (molecular non-metallic) and several black and grey forms (metalloids). Arsenic is found free in nature in three metalloidal forms with various crystal shapes (the minerals arsenopyrite and the considerably rarer arsenolamprite and pararsenolamprite), although it is more usually found as a combination with other elements. Aluminum is the most abundant metal in the earth’s crust, and it is always found in combination with other elements like oxygen, silicon, and fluorine.