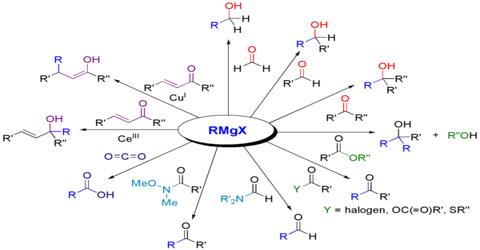
Application of Grignard Reagent: the Grignard reagent is a useful intermediate reagent in organic chemistry. The Grignard reaction is an organometallic chemical reaction in which alkyl, vinyl, or aryl-magnesium halides add to a carbonyl group in an aldehyde or ketone. By this reagent alkanes, alcohol, aldehydes, ketones, carboxylic acid could be prepared. A number of compounds produced by the Grignard reaction are very precious and unique intermediates or products in the field of pharmaceutical, fragrance, and other excellent or specialty chemicals.
A number of compounds produced by the Grignard reaction are very precious and unique intermediates or products in the field of pharmaceutical, fragrance, and other excellent or specialty chemicals.
These reagents were revealed by the French chemist Victor Grignard, who won the Nobel Prize in Chemistry in the year 1912 for his work on these compounds.
(i) Synthesis of hydrocarbon: Being hydrolyzed by water Grignard reagent forms hydrocarbons.
RMgX + H2O → R – H + Mg (OH) X
Example: CH3MgI + H2O → CH4 + Mg (OH) I
R-MgBr is the Grignard reagent. It forms this carbanion, R- (like CH3CH2-) This carb-anion can behave in two different ways. Further, the carb-anion can act as a nucleophilic and add into carbonyl groups with acid chlorides, esters, etc.
(ii) Synthesis of alcohol: Grignard reagent reacts with different carbonyl compounds forming an unstable transition stage of a compound which by hydrolysis forms different alcohols. The Grignard Reaction is the addition of an organo-magnesium halide (Grignard reagent) to a ketone or aldehyde, to form tertiary or secondary alcohol, respectively. The reaction with formaldehyde leads to primary alcohol.
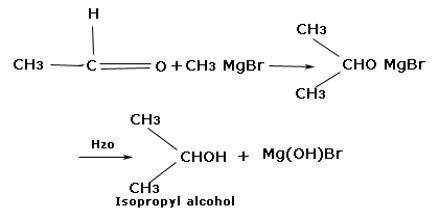
When methanal reacts with Grignard reagent, it forms primary alcohol. In the case of other aldehydes, secondary alcohols are formed. In the case of ketones, tertiary alcohols are formed.
Grignard reagents usually are organized by a reaction of an organ halogen with magnesium in a nitrogen atmosphere because the reagent is very immediate toward oxygen and moisture. Organ halogens differ significantly in their rates of reaction with magnesium. For example, alkyl iodides usually react exceedingly fast, whereas most aryl chlorides react gradually, if at all.
(iii) Grignard reagents and water:
Grignard reagents react with water to produce alkanes. This is the cause that everything has to be very dry during the preparation above.
For example:

The inorganic product, Mg(OH)Br, is referred to as a “basic bromide”. You can think of it as a sort of half-way stage between magnesium bromide and magnesium hydroxide.
(iv) Reaction with Acidic Hydrogens:
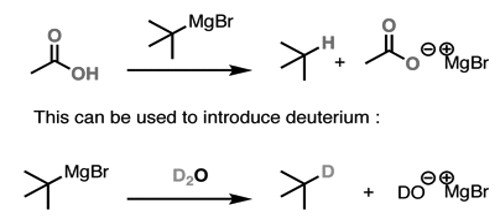
This can also be used to convert alkyl halides to alkanes. First, you care for it with magnesium, and then you indulgence the Grignard with a strong acid. This gives you the alkane. The first stride is to make the Grignard reagent. The second is to indulgence that Grignard with a deuterated acid such as D2O. This gives you the deuterated alkane.
Finally, Grignard reagents are enormously constructive organ metallic compounds in the field of organic chemistry. They show strong nucleophilic qualities and also have the capability to form new carbon-carbon bonds.