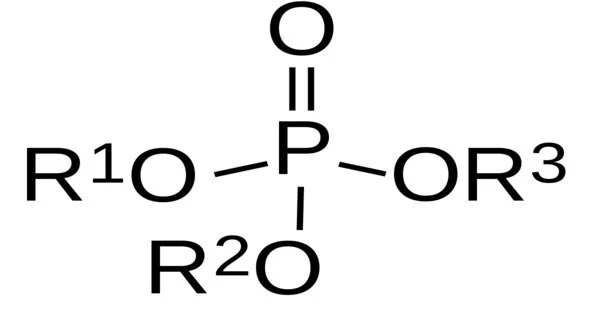
Boranophosphates are salts that contain an anion made up of borane (BH3) and phosphite groups. One of the most basic examples is [(CH3O)2OPBH3], which is produced by base hydrolysis of the borane-trimethylphosphite adduct.
Boranophosphates are actually a type of chemical compound that contain both boron and phosphorus. However, they do not contain borane (BH3) and phosphite groups. Borates are compounds that contain the BO3-3 ion, which consists of a central boron atom surrounded by three oxygen atoms. Boranes, on the other hand, are compounds that contain the BH3 molecule, which consists of a boron atom surrounded by three hydrogen atoms.
Boranophosphates are formed when borate and phosphate ions combine in a specific ratio. The resulting anion, BPO4-3, has one borate ion and three phosphate ions. This anion can then combine with various cations (positively charged ions) to form boranophosphate salts.
Boranophosphates are formed from the reaction of a boronic acid (a compound containing a boron atom bonded to an OH group) and a phosphite ester (a compound containing a phosphorus atom bonded to two OR groups). The resulting boranophosphate contains a boron atom bonded to two oxygen atoms (forming a BO2 group) and a phosphorus atom bonded to three oxygen atoms (forming a PO3 group). Boranophosphates have been studied for their potential use in various applications such as catalysis, materials science, and biomedicine due to their unique properties and structures.
Boranophosphates are being studied as structural surrogates for the phosphate group in living systems. Borane can replace one of the oxygens in a triphosphate or diphosphate functional group in some compounds. The phosphorus center is trivalent, but the phosphorus center remains tetrahedral.
Boranophosphates have been combined with nucleotides and investigated as potential therapeutic and diagnostic agents. They are one of several covalent phosphodi- and triester modifications. The compounds are made by attaching the borane to the phosphoramidite stage of nucleoside synthesis. The P-BH3 bond is strong enough to withstand deprotection methods, allowing the formation of the triphosphate.
Aside from boranophosphates, other di- and triphosphate modifications include phosphorothioates and methylphosphonate. They are also chiral for phosphorus.