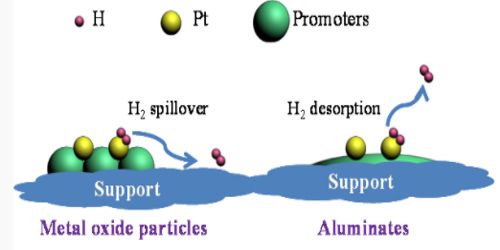
The role of promoters in Catalyst Adsorption
A small quantity of promoter is sufficient to increase the activity of the catalyst to a large extent. In Haber-Bosch synthesis of ammonia, small amounts of molybdenum, high melting oxides of some metals like aluminum, chromium, rare earths etc. are found to increase the activity of the iron catalyst considerably. Iron alone is not used but is always mixed with a suitable promoter. In the hydrogenation of vegetable oils the nickel catalyst is used along with promoters like copper and tellurium.
It is suggested that the promoter increases the surface area of the catalyst by reducing the “particle size” of the catalyst and also by increasing the number of active sites.
Chemical promoters improve the effectiveness of the catalyst often by altering the electron distribution at the surface. Structural promoters improve the mechanical properties and help prevent sintering. It is also possible that the catalyst and the promoter form a loose type of compound and this compound offers a better chemisorptions site for the reacting molecules. This may roughly explain the selectivity of the promoter action. It may also be possible that the catalyst-promoter pair offers competitive sites for adsorption to the molecules and the different atoms of the reacting molecules are chemisorbed on both the surfaces leaving the adsorbed molecules in a highly strained active form, thus enhancing the reaction rate.