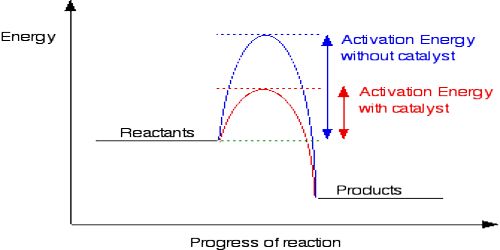
A catalyst is a chemical entity which by virtue of its presence in a reacting system increases or decreases the rate of the reaction, itself remaining unchanged in chemical properties or mass at the end of a reaction. The phenomenon of alteration of the rate of a reaction by a catalyst is known catalysis.
A catalyst which increases the rate of the reaction is said to be a positive catalyst. In most cases catalysts are used to increase the rate of reactions and our discussion will be mainly about positive catalysts. If, however, a catalyst decreases the rate of a reaction it is called a negative catalyst. Negative catalysts find application in controlling undesirable side reactions. In some reactions one of the products formed during the reaction acts as a positive catalyst for the overall reaction. Such a phenomenon is called autocatalysis.
When KMnO4 is slowly added to a solution of oxalic acid the pink colour of the permanganate is discharged slowly at first (reaction is slaw), but after a certain volume of the permanganate solution has been added the colour is discharged very rapidly (reaction is fast), i.e., some species formed as a result of the reaction act as catalyst for the reaction.
Types of Catalysis
Catalysis has been divided into three types:
- Homogeneous catalysis.
- Heterogeneous catalysis and
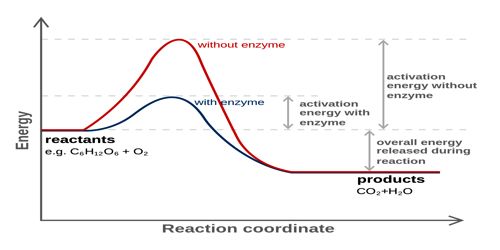
- Enzyme catalysis (Biological catalysts)