Homogeneous catalysis
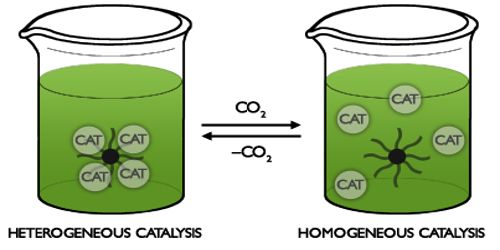
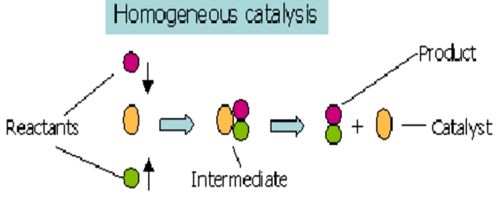
In a reaction, if the catalyst is present in the same phase as the reactants, it is called a homogeneous catalyst and the phenomenon is homogeneous catalysis. Such catalysis can take place in gaseous reaction or reactions in solution. This type of catalysis can sometimes be explained in terms of the formation of an intermediate compound, or ion or a radical. These chemicals help in attaining the equilibrium more quickly by increasing the rates of both the forward and reverse reactions to an extent. Acid catalysis, organometallic catalysis, and enzymatic catalysis are examples of homogeneous catalysis.
Examples of homogeneous catalysis in the gas phase are:
(a) Oxidation of sulphur dioxide, SO2, by oxygen to sulphur trioxide, SO3, in presence of nitric oxide, NO, in the Chamber Process for sulphuric acid manufacture.
2SO2 (g) + O2 (g) → 2SO3 (g)
Here NO acts as a catalyst.
(b) The following reaction in the gas phase is catalyzed by traces of chlorine gas, particularly in presence of tight.
2N2O (g) → 2N2 (g) + O2 (g)
In presence of light chlorine forms chlorine radicals, which react with N2O forming the intermediate radical ClO*. The proposed mechanism is:
- Step 1: N2O (g) + Cl* (g) → N2 (g) + ClO*(g)
- Step 2: 2ClO*(g) → Cl2 (g) + O2 (g)
(c) Some examples of homogeneous Catalysis in solution are as follows:
(i) Hydrolysis of ester in the presence of acid and alkali:
CH3COOC2H5 (l) + H2O (l) → CH3COOH (aq) + C2H5OH (aq)
(ii) Hydrolysis of sucrose (cane sugar) into glucose and fructose in presence of minerals acids acting as catalysts:
C12H22O11 (aq) (cane sugar) + H2O (l) → C6H12O6 (aq) (glucose) + C6H12O6 (aq) (fructose)
Most often, homogeneous catalysis involves the introduction of an aqueous phase catalyst into an aqueous solution of reactants. In such cases, acids and bases are often very effective catalysts, as they can speed up reactions by affecting bond polarization.
Applications
An advantage of homogeneous catalysis is that the catalyst mixes into the reaction mixture, allowing a very high degree of interaction between catalyst and reactant molecules. However, unlike with heterogeneous catalysis, the homogeneous catalyst is often irrecoverable after the reaction has run to completion.
Homogeneous catalysts are used in a variety of industrial applications, as they allow for an increase in reaction rate without an increase in temperature.
Homogeneous catalysis underwent a huge expansion in broadness and scope over the last two decades, so that it would be difficult to trace the boundaries of its current interest in, for example, organic synthesis, and polymer and pharmaceutical chemistry.