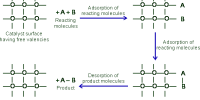
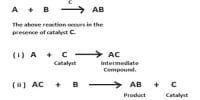
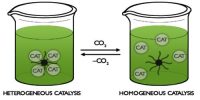
Catalytic promoter: Promoters are substances which when mixed with the catalyst increases its catalytic activity. These substances themselves are not catalysts for the reaction. A small quantity of promoter is sufficient to increase the activity of the catalyst to a large extent. A promoter is an accelerator of catalysis, but not a catalyst by itself. An inhibitor inhibits the working of a catalyst.
Few examples are:
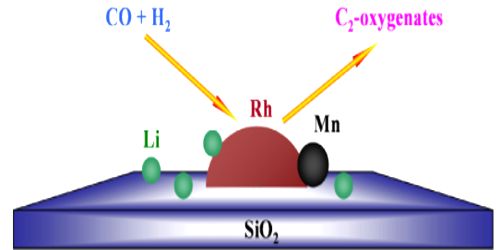
(i) In the Haber-Bosch process for the synthesis of ammonia, small amounts of molybdenum, high melting oxides of some metals like aluminum, chromium, rare earths etc. are found to increase the activity of the iron catalyst considerably. For this reason in industry iron alone is not used but is always mixed with a suitable promoter. For example, in Haber’s process for the synthesis of ammonia, traces of molybdenum increases the activity of finely divided iron which acts as a catalyst.
(ii) In the hydrogenation of vegetable oils the nickel catalyst is used along with promoters like copper and tellurium. In the manufacture of methyl alcohol from water gas, chromic oxide is used as a promoter with the catalyst zinc oxide.
By increasing the temperature, there is an increase in the catalytic power of a catalyst but after a certain temperature its power begins to decrease. A catalyst has thus, a particular temperature at which its catalytic activity is maximum.