A Solution is a homogeneous mixture of two (or more) pure substances and typically consists of a solvent (the “dissolver”) which is generally present in the larger amount and a solute (the substance being dissolved) which is generally present in the smaller amount.
Usually we are talking about a solid that dissolves in a liquid, but we could mean a gas dissolved in a liquid, or a liquid dissolved in a liquid.
The liquid is most often water – e.g. copper sulphate solution, or instant coffee. The solvent could be other liquids – e.g. methanol or cyclohexane.
Dissolving: When a solution is made, the bonds between the solute particles are broken, and they spread out throughout the solvent This is called dissolving. New bonds are formed between the solute and the solvent molecules.
E.g. H20 and NaCI(s) gives Ne+ (aq) + C1– (aq)
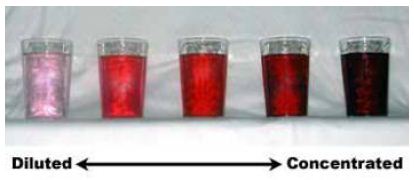
Concentration: The concentration of a solution can be defined as the amount of solute dissolved in a particular volume of solution. Therefore a dilute solution contains a low concentration of solute and a concentrated solution contains a high concentration of solute.
Typically, “dilute” and “concentrated” are general terms. For accurate and quantitative work, more precision is necessary.