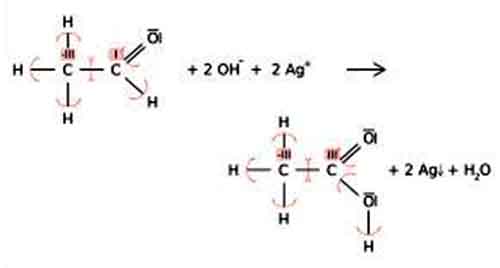
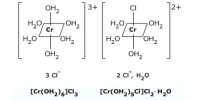
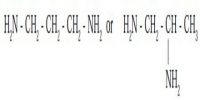Oxidation state: This number denotes the charge, explaining the number of electrons it has lost to form the cation. It is oxidation number that denotes the charge, if the central metal atom would have if all the ligand in the complex were removed along with their electron pairs that were shared with the central atom. It is usually represented by Roman Numeral.