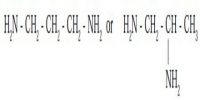A ligand is an ion (or) a molecule capable of functioning as an electron donor. Therefore the neutral molecules or ions which are directly attached to the central metal ion are called as ligand (or) coordination groups.
These coordination groups or ligands can donate a pair of electrons to the central metal ion (or) atom. Hence, in complex compound ligands act as Lewis bases. When a ligand is bound to a metal ion through a single donor atom, as with Cl–, H2O or NH3, the ligand is said to be unidentate.