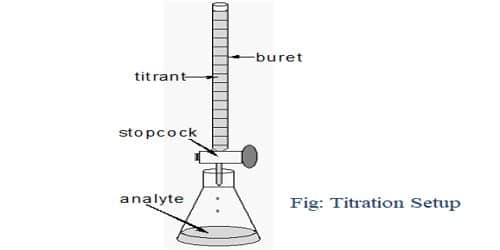
Principle of Titration: Titration is the process for the determination of the unknown concentration of a solution volumetrically. In this process, a solution of the primary standard substance of a particular volume is taken into a conical flask using the pipette. Then 1-2 drops of an indicator are added to it. The solution of unknown concentration is taken into the burette and added this solution to the conical flask for a reaction. After completing the reaction the color of the solution become changed. Then using the known volume of primary and secondary standard solution and the known concentration of a primary standard solution, the unknown concentration is determined.
The word titration comes from the Latin word “titulus”, which means inscription or title. It is a quantitative chemical examination. It is used to find out an unknown concentration of a known material in a sample. Therefore, Titration means the determination of concentration of a solution with esteem to water with a pH of 7. In simple logic, titration is a method to find out the concentration of an unknown solution.
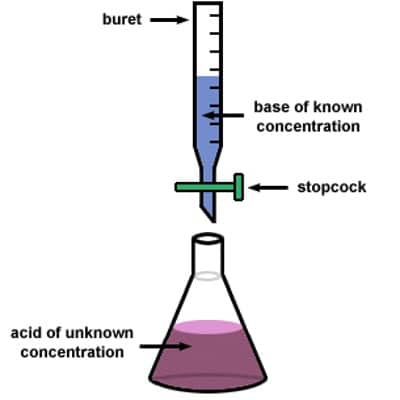
The fundamental principle of the titration is the following: A solution (titrant or standard solution) is added to the sample to be analyzed. The titrant contains a known concentration of a chemical that reacts with the material to be resolute. The titrant or standard solution is generally added from a graduated vessel called a burette. A burette is an apparatus that allows us to accurately measure the quantity (volume) of the titrant added. The procedure of adding titrant until the reaction is just entire is termed as titration and the material to be determined is said to be titrated. The solution called the titrant must convince the essential requirements to be a key or minor average.
All chemical reactions cannot be measured as titrations. A reaction can serve as a foundation of a titration process only if the following circumstances are fulfilled:
- The reaction must be a fast one.
- The change in free energy (ΔG) during the reaction must be adequately big for the impulsiveness of the reaction.
- There should be a method to notice the conclusion of the reaction.