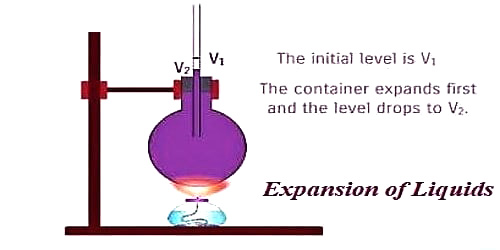
Liquid always has to be heated keeping it in a container. The liquid, as well as the container, expand when the heat is applied. A liquid is heated in a container. Heat flows from side to side the container to the liquid. This means that the container expands first, due to which the level of the liquid falls. So, the expansion of liquids observed by us is not the real expansion but it is an apparent expansion. Apparent Expansion means the thermal expansion of a liquid as measured in a graduated container without allowance for the expansion of the container.
There are two types of expansions of liquids
- Real expansion
- Apparent expansion
The real expansion of the liquid = Apparent expansion of the liquid + Volume expansion of the container.
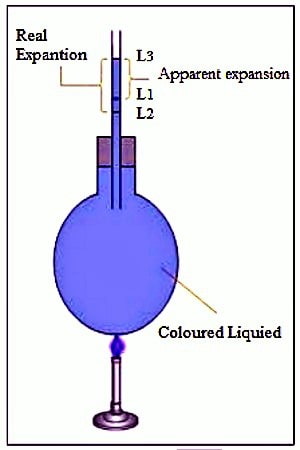
Real expansion: If it had been possible to heat a liquid without keeping it in a vessel then the real expansion of the liquid that would be obtained is called the real expansion of the liquid. But heating a liquid without a container is not possible. So, considering the expansion of the container the actual expansion of the liquid that is obtained is called the real expansion. It is expressed by Vr.
Apparent expansion: The expansion of liquid apparently observed without considering the expansion of the container is called the apparent expansion of the liquid. When the liquid gets heated, it expands further and supplementary than its original stage. We cannot monitor the middle state. We can only observe the primary and the last levels. This observed expansion of the liquid is known as the apparent expansion of the liquid and is less than its actual expansion. It is denoted by Va.
Explanation and differentiate the Real and Apparent Expansion of Liquids –
Any attempt at direct measurement of the growth of a liquid is problematical by the fact that the containing vessel itself expands. When a liquid is heated in a container, heat flows through the container to the liquid; which means that the container expands initial, due to which the stage of the liquid falls. The apparent broadly of a liquid is the portion of its volume by which the liquid appears to expand per Kelvin rise in temperature when heated in an expansible vessel. When the liquid gets heated, it expands more, beyond its original level. We cannot observe the intermediate state. This observed expansion of the liquid is known as the apparent expansion of the liquid. To estimate the apparent expansion, just fill a container until its brim with a given liquid and then heat the system. The volume poured out will be in the quantity of liquid that has apparently dilatated.
Since the expansion of liquid is complicated because of the expansion of the container, it is, therefore, necessary to distinguish between the real and apparent cubic expansively of liquid. If we consider the expansion of the container also and measure the total expansion in the volume of the liquid, then the expansion is termed as the real expansion of the liquid.