Nature of coordination (or) complex compounds
(i) An anionic complex compound contains a complex anion and simple cation.
K4[Fe(CN)6] ↔ 4K+ (simple cation) + [Fe (CN)6]4- (complex anion)
(ii) A cationic complex contains complex cation and simple anion
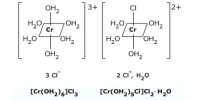
[Co(NH3)6] Cl3 ↔ [Co(NH3)6]3+ (complex cation) + 3Cl (simple anion)
(iii) In the case of a complex compound, [Cr(NH3)6] [Co(CN)6], it gives both complex cation and complex anion
[Cr(NH3)6] [Co(CN)6] ↔ [Cr(NH3)6]3+ (complex cation) + [Co(CN)6]3- (complex anion)