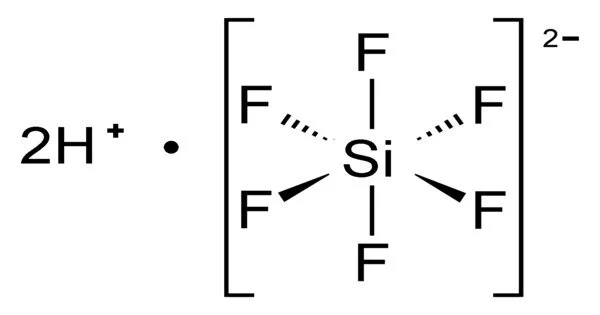
Hexafluorosilicic acid (H2SiF6) is an inorganic compound with the formula H2SiF6. Hexafluorosilicic acid aqueous solutions contain salts of the cation and the hexafluorosilicate anion. Colorless salts and aqueous solutions are produced by these salts.
Volcanoes produce a large amount of hexafluorosilicic acid naturally. It is produced as a byproduct of the phosphate fertilizer industry. The resulting hexafluorosilicic acid is almost entirely used as a precursor to aluminum trifluoride and synthetic cryolite, both of which are used in the aluminum processing process. Hexafluorosilicates are salts derived from hexafluorosilicic acid.
Production and principal reactions
Hexafluorosilicic acid is commercially produced from fluoride-containing minerals containing silicates. Apatite and fluorapatite are specifically treated with sulfuric acid to produce phosphoric acid, a precursor to several water-soluble fertilizers. This is referred to as the wet phosphoric acid process. As a byproduct of reactions involving silica-containing mineral impurities, approximately 50 kg of hexafluorosilicic acid is produced per tonne of HF.
It is commonly produced as a byproduct of the production of phosphate fertilizer or from the industrial processing of fluoride-containing minerals. Water fluoridation is a major application of hexafluorosilicic acid. It is used to supplement drinking water with fluoride ions in order to prevent tooth decay and improve dental health. The addition of hexafluorosilicic acid to water aids in the maintenance of an optimal fluoride concentration for dental benefits.
Properties
- Physical State: It is a liquid at room temperature. It has a boiling point of approximately 108 °C (226 °F).
- Solubility: It is highly soluble in water, and when dissolved, it forms a solution known as hexafluorosilicic acid solution. The solubility allows it to be easily handled and used in various applications.
- Acidity: It is a strong acid. In aqueous solutions, it dissociates to release hydrogen ions (H+) and hexafluorosilicate ions (SiF6-). The dissociation of the acid contributes to its corrosive properties.
- Corrosive Nature: It is highly corrosive to metals, glass, and other materials. It can cause severe burns and eye damage upon contact with the skin or eyes. Proper safety precautions, including the use of protective equipment, are necessary when handling this acid.
- Reactivity: It is a reactive compound. It reacts with various substances, including bases and metals, to produce different products. For example, it reacts with bases to form salts known as hexafluorosilicates. It can also release fluoride ions in certain reactions.
Uses:
Hexafluorosilicic acid has several applications. One of its primary uses is in water fluoridation, where it is added to public water supplies to help prevent tooth decay. It is also used in the production of aluminum, as a component in the manufacturing of ceramics and glass, and as a catalyst in certain chemical reactions.