Alzheimer’s disease is the most common and well-known tauopathy, which is a group of neurodegenerative brain illnesses characterized by toxic tangles of the protein tau. A study conducted by researchers at Washington University School of Medicine in St. Louis found that targeting astrocytes, a kind of inflammatory cell in the brain, lowers tau-related brain damage and inflammation in mice.
The findings, published online in Science Translational Medicine, underscore the critical function of astrocytes in causing brain damage in tauopathies and open up new paths toward better treatments for the group of catastrophic and difficult-to-treat illnesses.
“Brain inflammation is emerging as a factor to the development of Alzheimer’s disease, and that inflammation is caused by non-neuronal cells in the brain, including astrocytes,” said senior author and assistant professor of neurology Gilbert Gallardo, PhD. “Our findings show that inflammatory astrocytes contribute to tau-related diseases, and that lowering their reactivity may be advantageous in reducing brain inflammation and delaying Alzheimer’s progression.”
Our findings show that inflammatory astrocytes contribute to tau-related diseases, and that lowering their reactivity may be advantageous in reducing brain inflammation and delaying Alzheimer’s progression.
Gilbert Gallardo
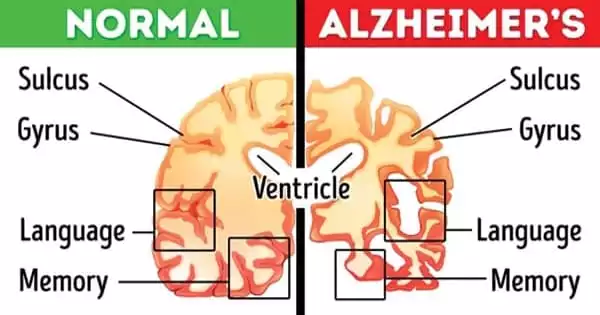
Tau, which is generally located inside neurons in the brain, aids in the formation of internal scaffolding that gives neurons their shape. When tau becomes knotted, it causes brain inflammation, tissue damage, and cognitive impairment. Tau tangles arise in people who have tau gene abnormalities or have been subjected to brain assaults such as repeated concussions or exposure to neurotoxic substances. Tau tangles appear relatively late in the Alzheimer’s disease process, likely prompted by earlier illness-related brain alterations such as the formation of amyloid beta plaques.
At regions of neuronal damage in many neurodegenerative disorders, so-called reactive astrocytes (astrocytes triggered in such a way that they cause harm to, rather than protect, brain tissue) are abundant. Gallardo and colleagues previously found an astrocyte protein that encouraged cells to take on toxic properties and worsen brain inflammation in amyotrophic lateral sclerosis (ALS), a neurodegenerative illness but not a tauopathy. Gallardo thought that the protein, known as alpha2-Na+/K+ adenosine triphosphatase (alpha2-NKA), was also responsible for astrocyte toxicity in Alzheimer’s disease and other tauopathies.
Gallardo is the first author. Carolyn Mann, a technician in Gallardo’s lab at the time, and co-author Celeste Karch, PhD, an associate professor of psychiatry, obtained data on the level of expression of the gene that codes for alpha2-NKA. They looked at brain samples from 80 people who died of Alzheimer’s disease, 82 people who died of a tauopathy known as progressive supranuclear palsy (PSP), and 76 people who died of causes unrelated to neurodegeneration. The researchers discovered that alpha2-NKA was more abundant in those who died from Alzheimer’s or PSP than in those who died from other causes, implying that the protein may be a contributor to brain damage in both disorders.
To further study the significance of alpha2-NKA, the researchers used mice that had been genetically modified to develop tau tangles by the age of 6 months. By 9 12 months of age, such animals’ brains are damaged, atrophied, and inflamed, and they have lost the capacity to conduct basic rodent functions like nest building. The researchers discovered that the genetically transformed mice exhibited higher amounts of alpha2-NKA in their brains, just like patients with tauopathies. The levels increased as the mice aged, worsening the inflammation and brain damage.
Digoxin, a medication used to treat cardiac problems, inhibits alpha2-NKA action. The researchers wanted to see if giving digoxin to mice would lessen tau tangles, brain shrinkage and inflammation, and behavioral abnormalities. The compound worked, and it worked whether it was given to mice under 6 months old, when the animals were just starting to form tau tangles, or at 8 months, when the tangles and damage were fully entrenched.
“The take-home lesson here is that reducing the inflammatory astrocytic state stops disease development,” Mann explained. “This is significant since experimental therapies for Alzheimer’s disease and related tauopathies have mostly focused on removing pathogenic proteins implicated in neuronal dysfunction and death. However, our findings suggest that focusing on inflamed astrocytes and brain inflammation may be the key to successfully treating such disorders.”
While digoxin has been approved by the FDA for specific cardiac diseases, its effects on the brain must be explored further before it can be considered as a potential therapy for Alzheimer’s and related tauopathies, according to Gallardo.