Obesity is becoming increasingly common around the world, but treatment options are limited. Despite initial success, calorie restriction (CR) weight loss frequently fails due to rebound weight gain. One direct cause of this undesirable outcome appears to be postdiet hyperphagia, which is associated with altered hypothalamic neuro-architecture. In response to calorie restriction, circulating levels of the appetite-stimulating hormone acyl-ghrelin rise dramatically.
Scientists reported that inhibiting a liver enzyme in obese mice resulted in promising results including reduced appetite and weight loss after 12 weeks of therapy. The research suggests a new way to intervene in metabolic diseases. Scientists from The University of Texas Health Science Center at San Antonio (UT Health San Antonio) reported today that inhibiting a liver enzyme in obese mice reduced the rodents’ appetite, increased energy expenditure in adipose (fat) tissues, and resulted in weight loss.
According to the authors, the discovery, published in Cell Metabolism, provides a potentially desirable drug target for treating metabolic issues such as obesity and diabetes. “We needed to discover this mechanism first, and now that we have, we can develop drugs to improve metabolic syndrome,” said senior author Masahiro Morita, PhD, assistant professor of molecular medicine at the Sam and Ann Barshop Institute for Longevity and Aging Studies at UT Health San Antonio.
Targeting mRNA is a relatively novel concept in the treatment of metabolic disease. It is a new thinking platform for how to treat this group of diseases.
Professor Nicolas Musi
“We have an enzyme inhibitor that we want to make more specific to increase its effects,” said first author Sakie Katsumura, DDS, PhD, postdoctoral fellow in the Morita laboratory.
The liver enzyme CNOT6L deadenylase inhibits messenger ribonucleic acids (mRNAs), which normally transport genetic instructions from the nucleus to sites in the cell where two liver proteins are produced.
Growth differentiation factor 15 (GDF15) is one of the proteins that sends signals to two regions of the hindbrain to control food intake. FGF21, on the other hand, sends signals to brown and white adipose tissues to increase energy expenditure. CNOT6L deadenylase inhibits GDF15 and FGF21 mRNA code-carrying, reducing these benefits.
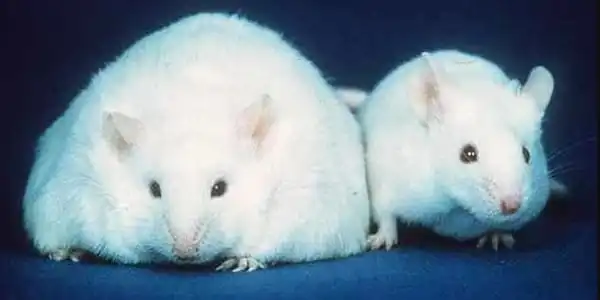
The researchers’ first-in-class CNOT6L inhibitor, dubbed iD1, stabilized GDF15 and FGF21 mRNAs in the liver of obese mice, increasing blood levels of the two proteins. After 12 weeks, treated rodents ate 40% less food and weighed 30% less. Adipose tissue energy expenditures increased by about 15%. The amount of fat in the liver was reduced by 30%.
iD1 treatment improved insulin sensitivity and reduced blood glucose levels in mice.
“Targeting mRNA is a relatively novel concept in the treatment of metabolic disease,” said coauthor Nicolas Musi, MD, professor of medicine at UT Health San Antonio and director of the Sam and Ann Barshop Institute. “It is a new thinking platform for how to treat this group of diseases.”
In Texas and the U.S., obesity, type 2 diabetes, fatty liver disease, and related metabolic disorders are at epidemic proportions.
Diabetes affects more than 37 million Americans, according to the Centers for Disease Control and Prevention (CDC). Type 2 diabetes accounts for at least 90% of all cases. Approximately 2.7 million people in Texas have diabetes, and an additional 600,000 have diabetes but are unaware of it. Prediabetes affects an additional 7 million people in Texas.
According to the CDC, the obesity rate in the United States is more than 40% and rising. Heart attacks, strokes, type 2 diabetes, and some cancers are all linked to obesity.
“These are very serious problems, and any intervention, including drugs, that can treat them is required,” Dr. Musi said. “Dr. Morita and Dr. Katsumura have made a groundbreaking discovery by delineating this mechanism and providing proof of concept that a drug targeting this pathway improves all of these parameters, including glucose levels, glucose tolerance, and insulin resistance caused by a high-fat diet and fatty liver.”
Dr. Katsumura reiterated that their next step is to refine this mechanism and identify new drugs that are more specific and potent.