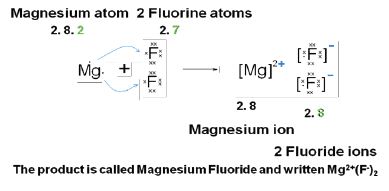
Drawing atoms and ions with all the orbits or orbitals is time consuming. We usually draw Lewis diagrams to represent the way that electrons form bonds. These are simpler and quicker. A Lewis structure can be drawn for any covalently bonded molecule, as well as coordination compounds.
Rules for drawing Lewis diagrams
Count the number of valence electrons each atom brings into the molecule. For ions, the charge must be taken into account. Write the symbol for the element at the centre. Draw a dot or a cross for each electron around the symbol – use dots for one element, and crosses for the other, to show which atoms the electrons have come from (although once the bond has been made, they are indistinguishable). Lewis diagrams show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule.