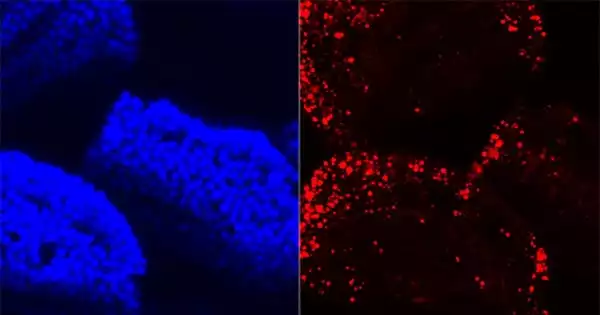
According to a new Duke study in mice, the microorganisms that assist break down food actually tell the gut how to do its job better. According to the researchers, bacteria appear to be able to control which of the gut’s genes are activated, and this interaction may lead to a remodeling of the epithelial cells lining the gut to match the diet.
“The gut is a wonderful link between an animal and the world it lives in, and it receives information from both the nutrition and the bacteria it houses,” said John Rawls, Ph.D., director of the Duke Microbiome Center and professor of molecular genetics and microbiology at Duke.
Cellular and Molecular Gastroenterology and Hepatology, an open access journal, published the findings. The Duke researchers contrasted mice reared without any gut microorganisms versus those raised with a normal gut microbiome to begin parsing the information going from the microbes to the cells of the gut. The researchers concentrated on the interaction between RNA transcription (the process by which DNA is transferred to RNA) and the proteins that turn this copying process on or off in the small intestine, which is where most fat and other nutrients are absorbed.
The gut is a wonderful link between an animal and the world it lives in, and it receives information from both the nutrition and the bacteria it houses. We were shocked to see that the gene playbook used by the gut epithelium to respond to dietary fat differs depending on whether or not microorganisms are present.
John Rawls
While both germ-free and normal mice could metabolize fatty acids in a high-fat diet, the researchers discovered that germ-free mice used a very distinct collection of genes to deal with the high-fat meal.
“We were shocked to see that the gene playbook used by the gut epithelium to respond to dietary fat differs depending on whether or not microorganisms are present,” Rawls said. The researchers also discovered that microorganisms can aid in fat absorption in the gut.
“It’s a really consistent conclusion across numerous studies, from our lab and others, that microorganisms actually improve lipid absorption,” said Colin Lickwar, Ph.D., senior research associate in Rawls’ lab and the paper’s first author. “And, to some extent, this influences systemic processes such as weight gain.”
The germ-free mice saw an increase in activity of the genes involved in fatty acid oxidation, literally burning of fatty acids, to provide fuel for the gut’s cells.
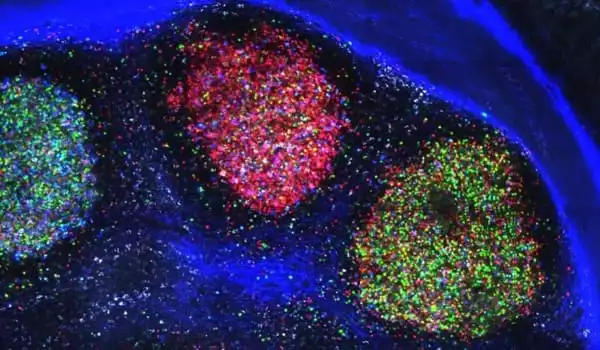
“Typically we think about the gut just doing its job absorbing dietary nutrients across the epithelium to share with the rest of the body, but the gut has to eat too,” Rawls said. “So what we think is going on in germ-free animals, is that the gut is consuming more of the fat than it would if the microbes were there.”
And that may reflect differences in the composition of the gut’s epithelial cells.
“There are a bunch of recent papers showing that there is a substantial capacity to change the larger architecture of the intestine as well as in the individual gene programs,” Lickwar said. “There is a remarkable amount of plasticity in the intestine. We largely don’t understand it, but some of it is elucidated by this paper.”
The researchers concentrated their efforts on a transcription factor known as HNF4-Alpha, which is known to regulate genes involved in lipid metabolism and genes that respond to microorganisms. “We felt it would represent an interface or a crossroads between understanding information from either microbial sources or dietary fat,” Lickwar said.
“It’s certainly difficult,” Lickwar said, “but we do appear to establish that HNF4-Alpha is critical in simultaneously integrating various signals throughout the intestine.”
“For every manner that germ-free animals appear strange, it teaches us something about the tremendous impact of the microbiome on what we assume to be ‘normal’ animal biology,” Rawls said.